Abstract
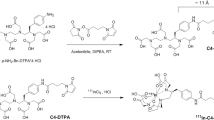
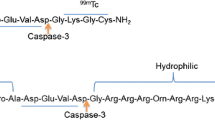
The aim of this study was to characterize the in vivo volumetric distribution of three folate-based biosensors by different imaging modalities (X-ray, fluorescence, Cerenkov luminescence, and radioisotopic imaging) through the development of a tridimensional image reconstruction algorithm. The preclinical and multimodal Xtreme imaging system, with a Multimodal Animal Rotation System (MARS), was used to acquire bidimensional images, which were processed to obtain the tridimensional reconstruction. Images of mice at different times (biosensor distribution) were simultaneously obtained from the four imaging modalities. The filtered back projection and inverse Radon transformation were used as main image-processing techniques. The algorithm developed in Matlab was able to calculate the volumetric profiles of 99mTc-Folate-Bombesin (radioisotopic image), 177Lu-Folate-Bombesin (Cerenkov image), and FolateRSense™ 680 (fluorescence image) in tumors and kidneys of mice, and no significant differences were detected in the volumetric quantifications among measurement techniques. The imaging tridimensional reconstruction algorithm can be easily extrapolated to different 2D acquisition-type images. This characteristic flexibility of the algorithm developed in this study is a remarkable advantage in comparison to similar reconstruction methods.










Similar content being viewed by others
References
Wang DS, Dake MD, Park JM, Kuo MD (2009) Molecular imaging: a primer for interventionalists and imagers. J Vasc Interv Radiol 20:1405–1423
Hoffman RM (2002) In vivo imaging of metastatic cancer with fluorescent proteins. Cell Death Differ 9(8):786–789. https://doi.org/10.1038/sj.cdd.4401077
Liu H, Ren G, Miao Z, Zhang X, Tang X, Han P et al (2010) Molecular optical imaging with radioactive probes. PLoS One 5(12):e14484. https://doi.org/10.1371/journal.pone.0014484
Stuker F, Ripoll J, Rudin M (2011) Fluorescence molecular tomography: principles and potential for pharmaceutical research. Pharmaceutics 3(4):229–274. https://doi.org/10.3390/pharmaceutics3020229
Rao J, Dragulescu-Andrasi A, Yao H (2007) Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol 18(1):17–25. https://doi.org/10.1016/j.copbio.2007.01.003
Ocak M, Gillman AG, Bresee J, Zhang L, Vlad AM, Müller C, Schibli R, Edwards WB, Anderson CJ, Gach HM (2015) Folate receptor-targeted multimodality imaging of ovarian cancer in a novel syngeneic mouse model. Mol Pharm 12(2):542–553. https://doi.org/10.1021/mp500628g
Aranda-Lara L, Ferro-Flores G, Ramírez F de M, Ocampo-García B, Santos-Cuevas C, Díaz-Nieto L et al. (2016) Improved radiopharmaceutical based on 99mTc-Bombesin–folate for breast tumour imaging. Nucl Med Commun [Internet]. 37:377–86. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006231-201604000-00007
Santos-Cuevas CL, Ferro-Flores G, Arteaga de Murphy C, Ramírez FDM, Luna-Gutiérrez MA, Pedraza-López M et al (2009) Design, preparation, in vitro and in vivo evaluation of 99mTc-N2S2-Tat(49-57)-bombesin: a target-specific hybrid radiopharmaceutical. Int J Pharm 375(1-2):75–83. https://doi.org/10.1016/j.ijpharm.2009.04.018
Kim SM, Choi N, Hwang S, Yim MS, Lee JS, Lee SM, Cho G, Ryu EK (2013) Folate receptor-specific positron emission tomography imaging with folic acid-conjugated tissue inhibitor of metalloproteinase-2. Bull Kor Chem Soc 34(11):3243–3248. https://doi.org/10.5012/bkcs.2013.34.11.3243
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK et al (2005) Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med 46(Suppl 1):62S–66S
Chu Z, La Sance K, Blanco V, Kwon C-H, Kaur B, Frederick M et al (2014) In vivo optical imaging of brain tumors and arthritis using fluorescent SapC-DOPS nanovesicles. J Vis Exp [Internet]. 12:1–7. Available from: http://www.jove.com/video/51187/in-vivo-optical-imaging-brain-tumors-arthritis-using-fluorescent-sapc
Aranda-Lara L, Ferro-Flores G, Azorin-Vega E, Ramirez FM, Jimenez-Mancilla N, Ocampo-Garcia B et al (2016) Synthesis and evaluation of Lys1(alpha,gamma-Folate)Lys3(177Lu-DOTA)-Bombesin(1–14) as a potential theranostic radiopharmaceutical for breast cancer. Appl Radiat Isot. Elsevier 107:214–219. https://doi.org/10.1016/j.apradiso.2015.10.030
Kelemen LE (2006) The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer 119(2):243–250. https://doi.org/10.1002/ijc.21712
Teng L, Xie J, Teng L, Lee RJ (2012) Clinical translation of folate receptor-targeted therapeutics. Expert Opin Drug Deliv 9:901–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22663189
Mitra A, Renukuntla J, Shah S, Boddu SHS, Vadlapudi A, Vadlapatla R, et al (2015) Functional characterization and expression of folate receptor-α in T47D human breast cancer cells. Drug Dev. Ther 6:52. Available from: http://www.ddtjournal.org/text.asp?2015/6/2/52/162441
Sancho V, Di Florio A, Moody TW, Jensen RT (2011) Bombesin receptor-mediated imaging and cytotoxicity: review and current status. Curr Drug Deliv 8(1):79–134. https://doi.org/10.2174/156720111793663624
Dalm SU, Martens JWM, Sieuwerts a. M, van Deurzen CHM, Koelewijn SJ, de Blois E, et al (2015) In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer. J Nucl Med 56:752–7. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.114.153023
Sasser ATA, Orton SP, Leevy MW (2014) Multimodal in vivo fluorescen, luminescent and X-ray imaging in preclinical studies of inflammation and immunobiology 2009–10
Bufkin K, Univversity W, Leevy M, Mentor PD (2015) Multimodal imaging trials with zebrafish specimens. 1:1–5
Martí-Bonmatí L, Sopena R, Bartumeus P, Sopena P (2010) Multimodality imaging techniques. Contrast Media Mol Imaging 5:180–9. Available from: http://doi.wiley.com/10.1002/cmmi.393
Magota K, Kubo N, Kuge Y, Nishijima K, Zhao S, Tamaki N (2011) Performance characterization of the Inveon preclinical small-animal PET/SPECT/CT system for multimodality imaging. Eur J Nucl Med Mol Imaging 38:742–752. https://doi.org/10.1007/s00259-010-1683-y
Hwang JY, Wachsmann-Hogiu S, Ramanujan VK, Ljubimova J, Gross Z, Gray HB et al (2012) A multimode optical imaging system for preclinical applications in vivo: Technology development, multiscale imaging, and chemotherapy assessment. Mol Imaging Biol 14:431–442. https://doi.org/10.1007/s11307-011-0517-z
Chapon C, Jackson JS, Aboagye EO, Herlihy AH, Jones WA, Bhakoo KK (2009) An in vivo multimodal imaging study using MRI and PET of stem cell transplantation after myocardial infarction in rats. Mol. Imaging Biol 11:31–38. https://doi.org/10.1007/s11307-008-0174-z
Paproski RJ, Li Y, Barber Q, Lewis JD, Campbell RE, Zemp R (2015) Validating tyrosinase homologue melA as a photoacoustic reporter gene for imaging Escherichia coli. J Biomed Opt 20:106008. Available from: http://biomedicaloptics.spiedigitallibrary.org/article.aspx?. https://doi.org/10.1117/1.JBO.20.10.106008
Doney E, Van Avermaete T, Chapman S, Waldeck J, Leevy WM (2013) Application note # AP0128 Jun 2013 Planar imaging of 99m Tc labeled SPECT probes in living mice using the In-Vivo Xtreme platform with radioisotopic phosphor Screen. 1–5
Sumi NJ, Lima E, Pizzonia J, Orton SP, Craveiro V, Joo W, et al (2014) Murine model for non-invasive imaging to detect and monitor ovarian cancer recurrence. J. Vis. Exp. [Internet]. e51815. Available from: http://www.jove.com/video/51815/murine-model-for-non-invasive-imaging-to-detect-monitor-ovarian
Hu GHG, Li HLH, Yao JYJ, Bai JBJ (2008) Fluorescent optical imaging of small animals using filtered back-projection 3D surface reconstruction method. 2008 Int Conf Biomed Eng Informatics 2:1000–1004
Kuo C, Coquoz O, Troy TL, Xu H, Rice BW (2007) Three-dimensional reconstruction of in vivo bioluminescent sources based on multispectral imaging. J Biomed Opt 12(2):024007. https://doi.org/10.1117/1.2717898
Song JS, Lee JM, Sohn JY, Yoon J-H, Han JK, Choi BI (2015) Hybrid iterative reconstruction technique for liver CT scans for image noise reduction and image quality improvement: evaluation of the optimal iterative reconstruction strengths. Radiol. Med 120:259–267. https://doi.org/10.1007/s11547-014-0441-9
Koch W, Suessmair C, Tatsch K, Pöpperl G (2011) Iterative reconstruction or filtered backprojection for semi-quantitative assessment of dopamine D2 receptor SPECT studies? Eur. J. Nucl. Med. Mol. Imaging 38:1095–1103. https://doi.org/10.1007/s00259-011-1737-9
Zeng GL (2010) Medical image reconstruction: a conceptual tutorial. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 125–173. https://doi.org/10.1007/978-3-642-05368-9_6
Kikuchi S, Matsuya A, Yamaguchi M, Ohyama N (1996) Microscopic computed tomography based on generalized analytic reconstruction from discrete samples. Opt Rev 3:22–28. https://doi.org/10.1007/s10043-996-0022-9
Aguirre J, Sisniega A, Ripoll J, Desco M, Vaquero JJ (2008) Design and development of a co-planar fluorescence and X-ray tomograph. IEEE Nucl Sci Symp Conf Rec 5412–3
Ducros N, Bassi A, Valentini G, Canti G, Arridge S, D’Andrea C (2013) Fluorescence molecular tomography of an animal model using structured light rotating view acquisition. J Biomed Opt 18(2):020503. https://doi.org/10.1117/1.JBO.18.2.020503
Liu X, Wang D, Bai J (2009) Fluorescence molecular tomography with optimal radon transform based surface reconstruction. Conf Proc IEEE Eng Med Biol Soc 2009:1404–1407. https://doi.org/10.1109/IEMBS.2009.5334178
Butz T. Fourier transformation for pedestrians. Cham: Springer International Publishing; 2015. p. 173–81. Available from: https://doi.org/10.1007/978-3-319-16985-9_7
Jähne B (1995) Digital image processing: concepts, algorithms, and scientific applications. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 231–252. https://doi.org/10.1007/978-3-662-03174-2_13
Prabhat P, Arumugam S, Madan VK (2012) Filtering in filtered backprojection computerized tomography. Proc NCNTE-2012 4–7
Kendziorra C, Meyer H, Dewey M (2015) Implementation of a phase detection algorithm for dynamic cardiac computed tomography analysis based on time dependent contrast agent distribution. PLoS one [internet]. Public Libr Sci 9:1–12. https://doi.org/10.1371/2Fjournal.pone.0116103
Hautière N, Tarel J-P, Aubert D, Dumont É (2008) Blind Contrast enhancement assessment by gradient ratioing at visible edges. Image Anal. Stereol. [Internet]. 27:87–95. Available from: http://www.ias-iss.org/ojs/IAS/article/view/834
Valencia-Murillo JF (2014) Poveda-Sendales DA, Valencia-Vargas DF. Evaluating the impact of image preprocessing on iris segmentation. Tecno Lógicas 17:31–41
Polesel A, Ramponi G, Mathews VJ (2000) Image enhancement via adaptive unsharp masking. IEEE Trans Image Process 9(3):505–510. https://doi.org/10.1109/83.826787
Ogoda M, Hishinuma K, Yamada M, Shimura K (1997) Unsharp masking technique using multiresolution analysis for computed radiography image enhancement. J Digit Imaging [Internet]. 10:185–9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3452800&tool=pmcentrez&rendertype=abstract
Suter SK, Ma B, Entezari A (2014) Advances in visual computing: 10th International Symposium, ISVC 2014, Las Vegas, NV, USA, December 8–10, 2014, Proceedings, Part I. In: Bebis G, Boyle R, Parvin B, Koracin D, McMahan R, Jerald J, et al., editors. Cham: Springer International Publishing. p. 313–22. Available from: https://doi.org/10.1007/978-3-319-14249-4_30
Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol 24:148–154. https://doi.org/10.1007/BF00300234
Schumann S, Liu L, Tannast M, Bergmann M, Nolte L-P, Zheng G (2013) An integrated system for 3D hip joint reconstruction from 2D X-rays: a preliminary validation study. Ann Biomed Eng 41:2077–2087. https://doi.org/10.1007/s10439-013-0822-6
Arranz A, Rudin M, Zaragoza C, Ripoll J (2015) Methods in mouse atherosclerosis. In: Andrés V, Dorado B, editors. New York, NY: Springer New York. p. 367–76. Available from: https://doi.org/10.1007/978-1-4939-2929-0_27
Backfrieder W, Hanel R, Diemling M, Lorang T, Kettenbach J, Imhof H (2001) Digital (R) evolution in radiology. In: Hruby W, editor. Vienna: Springer Vienna p. 131–9. Available from: https://doi.org/10.1007/978-3-7091-3707-9_16
Asaithambi N, Kayalvizhi R, Selvi W. Proceedings of the International Conference on Soft Computing Systems: ICSCS 2015, Volume 1. In: Suresh PL, Panigrahi KB, editors. New Delhi: Springer India; 2016. p. 415–25. Available from: https://doi.org/10.1007/978-81-322-2671-0_40
Chin PTK, Welling MM, Meskers SCJ, Valdes Olmos RA, Tanke H, Van Leeuwen FWB (2013) Optical imaging as an expansion of nuclear medicine: Cerenkov-based luminescence vs fluorescence-based luminescence. Eur J Nucl Med Mol Imaging 40(8):1283–1291. https://doi.org/10.1007/s00259-013-2408-9
Lee YC, Fullerton GD, Goins BA (2015) Comparison of multimodality image-based volumes in preclinical tumor models using In-Air micro-CT image volume as reference tumor volume. Open J. Med. Imaging [Internet]. 05:117–32. Available from: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=59462&#abstract
van Driel PB a. a., van de Giessen M, Boonstra MC, Snoeks TJ a., Keereweer S, Oliveira S, et al. (2014) Characterization and evaluation of the artemis camera for fluorescence-guided cancer surgery. Mol. Imaging Biol. [Internet]. 17:413–23. Available from: http://link.springer.com/10.1007/s11307-014-0799-z
Ferro-flores G, Ocampo-garcía BE, Santos-cuevas CL, María F De, Azorín-vega EP, Meléndez-alafort L (2015) Theranostic radiopharmaceuticals based on gold nanoparticles labeled with 177 Lu and conjugated to peptides 150–9
Jiménez-Mancilla N, Ferro-Flores G, Santos-Cuevas C, Ocampo-García B, Luna-Gutiérrez M, Azorín-Vega E, et al (2013) Multifunctional targeted therapy system based on (99m) Tc/(177) Lu-labeled gold nanoparticles-Tat(49–57)-Lys(3)-bombesin internalized in nuclei of prostate cancer cells. J. Labelled Comp. Radiopharm. [Internet]. 56:663–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25196028
Shrivastava A, Ding H, Kothandaraman S, Wang S-H, Gong L, Williams M et al (2014) A high-affinity near-infrared fluorescent probe to target bombesin receptors. Mol Imaging Biol 16:661–669. https://doi.org/10.1007/s11307-014-0727-2
Acknowledgements
The authors are grateful for the support of the Mexican National Council of Science and Technology (CONACYT-SEP-CB-2014-01-242443 and CONACyT-PDCPN-2015-01-1040) and to the National Polytechnic Institute (IPN-SIPCOFAA-2015-0344). This research was carried out as part of the activities of the “Laboratorio Nacional de Investigación y Desarrollo de Radiofármacos, CONACyT.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Ethical Committee for the Care and Use of Laboratory Animals (“Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán”).
Rights and permissions
About this article
Cite this article
Ramírez-Nava, G.J., Santos-Cuevas, C.L., Chairez, I. et al. Multimodal molecular 3D imaging for the tumoral volumetric distribution assessment of folate-based biosensors. Med Biol Eng Comput 56, 1135–1148 (2018). https://doi.org/10.1007/s11517-017-1755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-017-1755-2