Abstract
Visual inspection of electroencephalogram (EEG) recordings for epilepsy diagnosis is very time-consuming. Therefore, much research is devoted to developing a computer-assisted diagnostic system to relieve the workload of neurologists. In this study, a kernel version of the robust probabilistic collaborative representation-based classifier (R-ProCRC) is proposed for the detection of epileptic EEG signals. The kernel R-ProCRC jointly maximizes the likelihood that a test EEG sample belongs to each of the two classes (seizure and non-seizure), and uses the kernel function method to map the EEG samples into the higher dimensional space to relieve the problem that they are linearly non-separable in the original space. The wavelet transform with five scales is first employed to process the raw EEG signals. Next, the test EEG samples are collaboratively represented on the training sets by the kernel R-ProCRC and they are categorized by checking which class has the maximum likelihood. Finally, post-processing is deployed to reduce misjudgment and acquire more stable results. This method is evaluated on two EEG databases and yields an accuracy of 99.3% for interictal and ictal EEGs on the Bonn database. In addition, the average sensitivity of 97.48% and specificity of 96.81% are achieved from the Freiburg database.

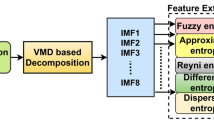
Visual inspection of EEG recordings for epilepsy diagnosis is very time-consuming. Therefore, many researchers are devoted to developing a computer-assisted diagnostic system to relieve the workload of neurologists. In this paper, a kernel version of the robust probabilistic collaborative representation based classifier (R-ProCRC) is proposed for the detection of epileptic EEG signals. The kernel R-ProCRC jointly maximizes the likelihood that a test EEG sample belongs to each of the two classes, i.e., seizure and non-seizure, and uses the kernel function method to map the EEG samples into the higher dimensional space to relieve the problem that they are linearly non-separable in the original space. The main procedures of the proposed method are exhibited in the two figures as following,
Fig. 1 The main procedures of the proposed method. (a) The schematic diagram of EEG classification based on the Freiburg database. (b) The detailed procedures of the kernel R-ProCRC
This method has been evaluated on two different types of EEG databases and shows superior performance.





Similar content being viewed by others
References
Fisher RS, WVE B, Blume W, Elger C, Genton P, Lee P, Engel J (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46(4):470–472. https://doi.org/10.1111/j.0013-9580.2005.66104.x
Behnam M, Pourghassem H (2017) Seizure-specific wavelet (Seizlet) design for epileptic seizure detection using CorrEntropy ellipse features based on seizure modulus maximas patterns. J Neurosci Methods:27684–27107
Zhang T, Chen W, Li M (2017) AR based quadratic feature extraction in the VMD domain for the automated seizure detection of EEG using random forest classifier. Biomed Signal Process:31550–31559
Acharya UR, Fujita H, Sudarshan VK, Bhat S, Koh JE (2015) Application of entropies for automated diagnosis of epilepsy using EEG signals: a review. Knowl-Based Syst:8885–8896
Gotman J (1982) Automatic recognition of epileptic seizures in the EEG. Electroencephalogr Clin Neurophysiol 54(5):530–540. https://doi.org/10.1016/0013-4694(82)90038-4
Aarabi A, Fazel-Rezai R, Aghakhani Y (2009) A fuzzy rule-based system for epileptic seizure detection in intracranial EEG. Clin Neurophysiol 120(9):1648–1657. https://doi.org/10.1016/j.clinph.2009.07.002
Orhan U, Hekim M, Ozer M (2011) EEG signals classification using the K-means clustering and a multilayer perceptron neural network model. Expert Syst Appl 38(10):13475–13481. https://doi.org/10.1016/j.eswa.2011.04.149
Acharya UR, Sree SV, Suri JS (2011) Automatic detection of epileptic EEG signals using higher order cumulant features. Int J Neural Syst 21(05):403–414. https://doi.org/10.1142/S0129065711002912
Srinivasan V, Eswaran C, Sriraam N (2007) Approximate entropy-based epileptic EEG detection using artificial neural networks. IEEE Trans Inf Technol Biomed 11(3):288–295. https://doi.org/10.1109/TITB.2006.884369
Übeyli ED (2010) Lyapunov exponents/probabilistic neural networks for analysis of EEG signals. Expert Syst Appl 37(2):985–992. https://doi.org/10.1016/j.eswa.2009.05.078
Yuan Q, Zhou W, Zhang L, Zhang F, Xu F, Leng Y, Wei D, Chen M (2017) Epileptic seizure detection based on imbalanced classification and wavelet packet transform. Seizure:5099–5108
Kıymık MK, Güler IN, Dizibüyük A, Akın M (2005) Comparison of STFT and wavelet transform methods in determining epileptic seizure activity in EEG signals for real-time application. Comput Biol Med 35(7):603–616. https://doi.org/10.1016/j.compbiomed.2004.05.001
Tzallas AT, Tsipouras MG, Fotiadis DI (2009) Epileptic seizure detection in EEGs using time–frequency analysis. IEEE Trans Inf Technol Biomed 13(5):703–710. https://doi.org/10.1109/TITB.2009.2017939
Subasi A (2007) EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert Syst Appl 32(4):1084–1093. https://doi.org/10.1016/j.eswa.2006.02.005
Wright J, Yang AY, Ganesh A, Sastry SS, Ma Y (2009) Robust face recognition via sparse representation. IEEE Trans Pattern Anal Mach Intell 31(2):210–227. https://doi.org/10.1109/TPAMI.2008.79
Zhang L, Yang M, Feng X (2011) Sparse representation or collaborative representation: which helps face recognition? In: IEEE Int Conf Computer Vision. p. 471–478
Yuan S, Zhou W, Yuan Q, Li X, Wu Q, Zhao X, Wang J (2015) Kernel collaborative representation-based automatic seizure detection in intracranial EEG. Int J Neural Syst 25(02):1550003. https://doi.org/10.1142/S0129065715500033
Yuan Q, Zhou W, Yuan S, Li X, Wang J, Jia G (2014) Epileptic EEG classification based on kernel sparse representation. Int J Neural Syst 24(04):1450015. https://doi.org/10.1142/S0129065714500154
Cai S, Zhang L, Zuo W, Feng X (2016) A probabilistic collaborative representation based approach for pattern classification. In: IEEE Int Conf on Computer Vision and Pattern Recognition. p. 2950–2959
Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE (2001) Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys Rev E 64(6):061907. https://doi.org/10.1103/PhysRevE.64.061907
Maiwald T (2004) Comparison of three nonlinear seizure prediction methods by means of the seizure prediction characteristic. Physica D 194(3):357–368
Kalayci T, Ozdamar O (1995) Wavelet preprocessing for automated neural network detection of EEG spikes. IEEE Eng Med Biol Mag 14(2):160–166. https://doi.org/10.1109/51.376754
Khan Y, Gotman J (2003) Wavelet based automatic seizure detection in intracerebral electroencephalogram. Clin Neurophysiol 114(5):898–908. https://doi.org/10.1016/S1388-2457(03)00035-X
Majumdar KK, Vardhan P (2011) Automatic seizure detection in ECoG by differential operator and windowed variance. IEEE Trans Neural Syst Rehab Eng 19(4):356–365. https://doi.org/10.1109/TNSRE.2011.2157525
Majumdar K (2012) Differential operator in seizure detection. Comput Biol Med 42(1):70–74. https://doi.org/10.1016/j.compbiomed.2011.10.010
Liu Q (2016) Kernel local sparse representation based classifier. Neural Process Lett 43(1):85–95. https://doi.org/10.1007/s11063-014-9403-4
Yang S, Han Y, Zhang X (2012) A sparse kernel representation method for image classification. In: (IJCNN). p. 1–7, DOI: https://doi.org/10.1007/s00253-018-9238-4
Temko A, Thomas E, Marnane W, Lightbody G, Boylan G (2011) EEG-based neonatal seizure detection with support vector machines. Clin Neurophysiol 122(3):464–473. https://doi.org/10.1016/j.clinph.2010.06.034
Raghunathan S, Jaitli A, Irazoqui PP (2011) Multistage seizure detection techniques optimized for low-power hardware platforms. Epilepsy Behav:22S61–22S68. https://doi.org/10.1016/j.yebeh.2011.09.008
Nigam VP, Graupe D (2004) A neural-network-based detection of epilepsy. Neurol Res 26(1):55–60
Guo L, Rivero D, Seoane JA, Pazos A (2009) Classification of EEG signals using relative wavelet energy and artificial neural networks. In: Proceedings of the first ACM/SIGEVO Summit on Genetic and Evolutionary Computation: ACM. p. 177–184
Tzallas A, Karvelis P, Katsis C, Fotiadis D, Giannopoulos S, Konitsiotis S (2006) A method for classification of transient events in EEG recordings: application to epilepsy diagnosis. Meth Inf Med 45(6):610–621
Wang Y, Zhou W, Yuan Q, Li X, Meng Q, Zhao X, Wang J (2013) Comparison of ictal and interictal EEG signals using fractal features. Int J Neural Syst 23(06):1350028. https://doi.org/10.1142/S0129065713500287
Yuan Q, Zhou W, Li S, Cai D (2011) Epileptic EEG classification based on extreme learning machine and nonlinear features. Epilepsy Res 96(1):29–38
Ocak H (2009) Automatic detection of epileptic seizures in EEG using discrete wavelet transform and approximate entropy. Expert Syst Appl 36(2):2027–2036. https://doi.org/10.1016/j.eswa.2007.12.065
Guo L, Rivero D, Dorado J, Rabunal JR, Pazos A (2010) Automatic epileptic seizure detection in EEGs based on line length feature and artificial neural networks. J Neurosci Methods 191(1):101–109. https://doi.org/10.1016/j.jneumeth.2010.05.020
Funding
This work was jointly financially supported by the National Natural Science Foundation of China (No. 61501283, No. 61701279, No. 61701270 and No. 61401259), the Shandong Provincial Natural Science Foundation (No. ZR2015PF012 and No. ZR2017PF006), and the China Postdoctoral Science Foundation (No. 2015 M582129 and No. 2015 M582128).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Z., Zhou, W., Zhang, F. et al. Automatic seizure detection based on kernel robust probabilistic collaborative representation. Med Biol Eng Comput 57, 205–219 (2019). https://doi.org/10.1007/s11517-018-1881-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-018-1881-5