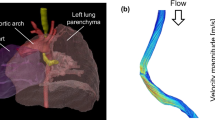
Abstract
Tracheal flow in infants with congenital tracheal stenosis (CTS) was numerically investigated using subject-specific airway models before and after reconstructive surgery. We quantified tracheal flow based on airway resistance during inhalation, and compared it between controls and patients before and after surgery. The airway resistance in each subject was assessed using geometrical parameters of the trachea: the minimum cross-sectional area Amin, the minimum cross-sectional area normalized by the standard deviation of the cross-sectional area Amin/σA, the area ratio of the minimum and maximum cross-sectional area Amin/Amax, and ratio of the normalized standard deviation of cross-sectional area to the mean cross-sectional area σA/Amean. Our numerical results demonstrated that such geometrical parameters could be used to assess the severity of CTS. Since subjects can be more clearly categorized as controls and most preoperative patients in terms of the airway resistance, a simulation using subject-specific airway models can lead us to a precise understanding of tracheal flow, and also provide knowledge about therapeutic decision. Our numerical results also demonstrated that significant surgical expansion of cross-sectional area did not help recover tracheal flow because of expansion loss. These results will be helpful not only when making therapeutic decisions about surgery but also when assessing quality of life in postoperative patients.

Graphical abstract







Similar content being viewed by others
References
Bates AJ, Comerford A, Cetto R, Schroter RC, Tolley NS, Doorly DJ (2016) Power loss mechanisms in pathological tracheas. J Biomech 49:2187–2192. https://doi.org/10.1016/j.jbiomech.2015.11.033
Brouns M, Jayaraju ST, Lacor C, De Mey J, Noppen M, Vincken W, Verbanck S (2007) Tracheal stenosis: a flow dynamics study. J Appl Physiol 103:1178–1184. https://doi.org/10.1152/japplphysiol.01063.2006.
Butler CR, Speggiorin S, Rijnberg FM, Roebuck DJ, Muthialu N, Hewitt RJ, Elliott MJ (2014) Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg 147:1783–1790. https://doi.org/10.1016/j.jtcvs.2014.02.069
Cheng W, Manson DE, Forte V, Ein SH, MacLusky L, Papsin BC, Hechter S, Kim PCW (2006) The role of conservative management in congenital tracheal stenosis: an evidence-based long-term follow-up study. J Pediatr Surg 41:1203–1207. https://doi.org/10.1016/j.jpedsurg.2006.03.046
Chiu PP, Kim PC (2006) Prognostic factors in the surgical treatment of congenital tracheal stenosis: a multicenter analysis of the literature. J Pediatr Surg 41:221–225. https://doi.org/10.1016/j.jpedsurg.2005.10.043
Clements JA, Brown ES, Johnson RP (1958) Pulmonary surface tension and the mucus lining of the lungs: some theoretical considerations. J Appl Physiol 12:262–268. https://doi.org/10.1152/jappl.1958.12.2.262
Davis PJ, Cladis FP (2016) Smith’s anesthesia for infants and children, 9th edn. Elsevier, Philadelphia
Ford S, Calvert J (2008) Adaptation for life: a review of neonatal physiology. Anaesth Intensive Care 9:93–98. https://doi.org/10.1016/j.mpaic.2008.01.009
Goldsmith JP, Karothin EH, Martin K, Gautham S (2016) Assisted ventilation of the neonate: evidence-based approach to newborn respiratory care, 6th edn. Elsevier, Philadelphia
Grinnan DC, Truwit JD (2005) Clinical review: respiratory mechanics in spontaneous and assisted ventilation. Crit Care 9:472–484. https://doi.org/10.1186/cc3516.
Heil M, Lazel AL (2011) Fluid-structure interaction in internal physiological flows. Annu Rev Fluid Mech 43:141–162. https://doi.org/10.1146/annurev-fluid-122109-160703
Herrera P, Caldarone C, Forte V, Campisi P, Holtby H, Chait P, Chiu P, Cox P, Yoo SJ, Manson D, Kim PC (2007) The current state of congenital tracheal stenosis. Pediatr Surg Int 23:1033–1044. https://doi.org/10.1007/s00383-007-1945-3
Hofferberth SC, Watters K, Rahbar R, Fynn-Thompson F (2015) Management of congenital tracheal stenosis. Pediatrics 136:e660–e669. https://doi.org/10.1542/peds.2014-3931
Kamm RD, Schroter RC (1989) Is airway closure caused by a liquid film instability? Respir Physiol 75:141–156. https://doi.org/10.1016/0034-5687(89)90059-5
Kleinstreuer C, Zhang Z (2010) Airflow and particle transport in the human respiratory system. Annu Rev Fluid Mech 42:301–334. https://doi.org/10.1146/annurev-fluid-121108-145453
Koshiyama K, Nishimoto K, Ii S, Sera T, Wada S (2018) Heterogeneous structure and surface tension effects on mechanical response in pulmonary acinus: a finite element analysis. Clin Biomech. https://doi.org/10.1016/j.clinbiomech.2018.01.001
Ma B, Lutchen KR (2009) CFD simulation of aerosol deposition in an anatomically based human large-medium airway model. Ann Biomed Eng 37:271–285. https://doi.org/10.1007/s10439-008-9620-y
Macklem PT (1971) Airway obstruction and collateral ventilation. Physiol Rev 51:368–436. https://doi.org/10.1152/physrev.1971.51.2.368
Manning PB, Rutter MJ, Lisec A, Gupta R, Marino BS (2011) One slide fits all: the versatility of slide tracheoplasty with cardiopulmonary bypass support for airway reconstruction in children. J Thorac Cardiovasc Surg 141:155–161. https://doi.org/10.1016/j.jtcvs.2010.08.060
Miki T, Wang X, Aoki T, Imai Y, Ishikawa T, Takase K, Yamaguchi T (2012) Patient-specific modeling of pulmonary air flow using GPU cluster for the application in medical particle. Comput Methods Biomech Biomed Eng 15:771–778. https://doi.org/10.1080/10255842.2011.560842.
Mimouni-Benabu O, Meister L, Giordano J, Fayoux P, Loundon N, Triglia JM, Nicollas R (2012) A preliminary study of computer assisted evaluation of congenital tracheal stenosis: a new tool for surgical decision-making. Int J Pediatr Otorhinolaryngol 76:1552–1557. https://doi.org/10.1016/j.ijporl.2012.07.009
Morita K, Yokoi A, Fukuzawa H, Hisamatsu C, Endo K, Okata Y, Tamaki A, Mishima Y, Oshima Y, Maeda K (2016) Surgical intervention strategies for congenital tracheal stenosis associated with a tracheal bronchus based on the location of stenosis. Pediatr Surg Int 32:915–919. https://doi.org/10.1007/s00383-016-3928-8
Otani T, Al-Issa A, Pourmorteza A, McVeigh ER, Wada S, Ashikaga H (2016) A computational framework for personalized blood flow analysis in the human left atrium. Ann Biomed Eng 44:3284–3294. https://doi.org/10.1007/s10439-016-1590-x
Pedley TJ, Schroter R, Sudlow M (1970) The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir Physiol 9:387–405. https://doi.org/10.1016/0034-5687(70)90094-0
Pedley TJ (1977) Pulmonary fluid dynamics. Ann Rev Fluid Mech 9:229–274. https://doi.org/10.1146/annurev.fl.09.010177.001305
Taherian S, Rahai HR, Bonifacio J, Gomez BZ, Waddington T (2018) Particulate deposition in a patient with tracheal stenosis. J Eng Sci Med Diagn Ther 1:011005. https://doi.org/10.1115/1.4038260
Yokoi A, Hasegawa T, Oshima Y, Higashide S, Nakatani E, Kaneda H, Kawamoto A, Nishijima E (2018) Clinical outcomes after tracheoplasty in patients with congenital tracheal stenosis in 1997-2004. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2017.12.017
Acknowledgments
This research was supported by JSPS KAKENHI Grant Number JP17K13015, and by the Keihanshin Consortium for Fostering the Next Generation of Global Leaders in Research (K-CONNEX), established by the Human Resource Development Program for Science and Technology, and also by Ministry of Education, Culture, Sports, Science, and Technology (MEXT) as “Priority Issue on post-K computer” (Integrated Computational Life Science to Support Personalized and Preventive Medicine) (Project ID: hp180202).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
1.1 Effects of length of straight tube and mesh resolution
We used various lengths of straight tubes (Ltube = 0, 10, 50, and 100 mm) at Re = 1000 with the normal real airway model of C1, and calculated the error of the maximum velocity at the start of the real airway, Uerr, as:
where U0, max is the maximum velocity at the start of the real airway for different Ltube (= 0, 10, and 50 mm) and \( {U}_{0,\mathit{\max}}^{ref} \) is the reference with the maximum straight tube length Ltube = 100 mm. The results of Uerr, presented in Fig. 8a, show that there was a less than 2% difference for Ltube = 50 mm and Ltube = 100 mm. Hence, we generally used 50-mm straight tubes in simulation, except for the highest Re (= 2000), where 100-mm straight tubes were used.
a The error of maximum velocity at the start of the real airway \( {U}_{err}=\mid {U}_{0,\mathit{\max}}-{U}_{0,\mathit{\max}}^{ref}\mid /{U}_{0,\mathit{\max}}^{ref} \) at Re = 1000, where U0,max is the maximum velocity for different Ltube (= 0, 10, and 50 mm), and \( {U}_{0,\mathit{\max}}^{ref} \) is the reference with a maximum tube length Ltube = 100 mm. bUerr is also calculated with the results of different mesh resolutions Δx, where \( {U}_{0,\mathit{\max}}^{ref} \) is the reference with the finest mesh resolution Δx = 0.125 mm with Ltube = 50 mm. These results were calculated with the normal real airway model of C1
To check the effect of the computational mesh resolution Δx, we performed simulations and calculated Uerr for different values of Δx (0.125, 0.25, 0.5, and 1 mm). The results of Uerr for different Δx are shown in Fig. 8b, where the reference is the result of the finest mesh resolution Δx = 0.125 mm. Since there was less than 0.4% difference between Δx = 0.25 and 0.125 mm, we defined the mesh size as Δx = 0.25 mm in our simulations.
1.2 Pressure gradient in subject-specific airway model
Assuming that the flow in an ideal straight tube is Poiseuille flow, the pressure gradient ΔP/ΔL can be expressed by:
where f is the coefficient (= 64/Re), U is the mean velocity, and D is the tube diameter. Using D = 2(A/π)1/2 and U = μRe/(ρD), the Eq. 5 is rewritten as follows:
The numerical result of ΔP/ΔL was calculated as follows:
where ΔP1in is the pressure difference between the inlet and branch point, and ΔP2out and ΔP3out are the pressure difference between the branch point and each outlet. ΔLi (i = 1–3) includes the length of the straight tubes, where ΔL1 is the length of the trachea before the branch point, and ΔL2 and ΔL3 are the lengths of the bronchi after the branch point. Figure 9 shows a comparison of ΔP/ΔL at relatively low Re (= 100 and 500) for the numerical and analytical results given by Eq. 6. As expected, the numerical values for pressure difference increase as the cross-sectional area decreases, independent of subject group. The pressure gradient was minimized when the airway was assumed to be a straight tube, and hence the numerical results of ΔP/ΔL were always plotted above the analytical solutions of Eq. 6 Through these analyses, we confirmed that approximate pressure gradient in a realistic airway model can be estimated by assuming a Poiseuille flow at least for Re ≤ 500.
Pressure gradient ΔP/ΔL as a function of mean cross-sectional area Amean for each subject type at Re = 100 (a) and 500 (b). The solid line is determined by Eq. (6)
Rights and permissions
About this article
Cite this article
Takeishi, N., Miki, T., Otani, T. et al. Fluid dynamic assessment of tracheal flow in infants with congenital tracheal stenosis before and after surgery. Med Biol Eng Comput 57, 837–847 (2019). https://doi.org/10.1007/s11517-018-1928-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-018-1928-7