Abstract
Metal implants are widely used in the treatment of orthopedic diseases. However, owing to the mismatched elastic modulus of the bone and implants, stress shielding often occurs clinically which can result in failure of the implant or fractures around the implant. Topology optimization (TO) is a technique that can provide more efficient material distribution according to the objective function under the special load and boundary conditions. Several researchers have paid close attention to TO for optimal design of orthopedic implants. Thanks to the development of additive manufacturing (AM), the complex structure of the TO design can be fabricated. This article mainly focuses on the current stage of TO technique with respect to the global layout and hierarchical structure in orthopedic implants. In each aspect, diverse implants in different orthopedic fields related to TO design are discussed. The characteristics of implants, methods of TO, validation methods of the newly designed implants, and limitations of current research have been summarized. The review concludes with future challenges and directions for research.
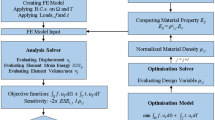
Graphical abstract

Wang TO design of global layout and local structure of implants in diverse fields of orthopedic







Similar content being viewed by others
References
Denard PJ, Raiss P, Gobezie R, Edwards TB, Lederman E (2018) Stress shielding of the humerus in press-fit anatomic shoulder arthroplasty: review and recommendations for evaluation. J Shoulder Elb Surg 27(6):1139–1147. https://doi.org/10.1016/j.jse.2017.12.020
Teles AR, Yavin D, Zafeiris CP, Thomas KC, Lewkonia P, Nicholls FH, Swamy G, Jacobs WB (2018) Fractures after removal of spinal instrumentation: revisiting the stress-shielding effect of instrumentation in spine fusion. World Neurosurg 116:e1137–e1e43. https://doi.org/10.1016/j.wneu.2018.05.187
Al-Tamimi PC Fernandes PR, editors. Topology optimization to reduce the stress shielding effect for orthopedic applications. Procedia CIRP 65:202–206. https://doi.org/10.1016/j.procir.2017.04.032
Arabnejad S, Johnston B, Tanzer M, Pasini D (2017) Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J Orthop Res 35(8):1774–1783. https://doi.org/10.1002/jor.23445
RS EKI, Nomura S (2005) Influence of marginal bone resorption on stress around an implant – a three-dimensional finite element analysis. J Oral Rehabil 32:279–286. https://doi.org/10.1111/j.1365-2842.2004.01413.x
Kuroda D, Niinomi M, Morinaga M, Kato Y, Yashiro T (1998) Design and mechanical properties of new β type titanium alloys for implant materials. MAT SCI ENG R: A 243(1):244–249. https://doi.org/10.1016/S0921-5093(97)00808-3
Saravana Kumar G, George SP (2017) Optimization of custom cementless stem using finite element analysis and elastic modulus distribution for reducing stress-shielding effect. Proc Inst Mech Eng H J Eng Med 231(2):149–159. https://doi.org/10.1177/0954411916686125
Bendsøe MP, Sigmund O 2003 Topology optimization - theory, methods, and applications: Springer Verlag
Zhu J-H, Zhang W-H, Xia L (2015) Topology optimization in aircraft and aerospace structures design. Arch Computat Methods Eng 23(4):595–622. https://doi.org/10.1007/s11831-015-9151-2
Bendsøe MP, Kikuchi N (1988) Generating optimal topologies in structural design using a homogenization method. COMPUT METHOD APPL M 71(2):197–224. https://doi.org/10.1016/0045-7825(88)90086-2
Wang X, Xu S, Zhou S, Xu W, Leary M, Choong P, Qian M, Brandt M, Xie YM (2016) Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials 83:127–141. https://doi.org/10.1016/j.biomaterials.2016.01.012
Brekelmans WAMRE, Burdeaux BD (1972) A new method to analyse the mechanical behaviour of skeletal parts. Acta Orthop Scand 43:301–317. https://doi.org/10.3109/17453677208998949
R CGSPK 2010 Oct. editor Application of topology optimization in modern additive manufacturing. 21st Annual International Solid Freeform Fabrication Symposium - An Additive Manufacturing Conference
Li Q, Steven GP, Xie YM (1999) On equivalence between stress criterion and stiffness criterion in evolutionary structural optimization. Structural optimization 18(1):67–73. https://doi.org/10.1007/BF01210693
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4:518–524. https://doi.org/10.1038/nmat1421
Bends M (1989) Optimal shape design as a material distribution problem Struct. Optimization 3:193–202. https://doi.org/10.1007/BF01650949
Cregoire AFJ, Anca-Maria T (2002) A level-set method for shape optimization. CR MATH 334:1125–1130 S1631-073X(02)02412-3
Fan Z, Xia L, Lai W, Xia Q, Shi T (2019) Evolutionary topology optimization of continuum structures with stress constraints. Struct Multidiscip Optim 59(2):647–658. https://doi.org/10.1007/s00158-018-2090-4
Svanberg K (1987) The method of moving asymptotes—a new method for structural optimization. Int J Numer Methods Eng 24(2):359–373. https://doi.org/10.1002/nme.1620240207
Xie YM, Steven GP (1993) A simple evolutionary procedure for structural optimization. Comput Struct 49(5):885–896. https://doi.org/10.1016/0045-7949(93)90035-C
Xue R, Liu C, Zhang W, Zhu Y, Tang S, Du Z, Guo X (2019) Explicit structural topology optimization under finite deformation via Moving Morphable Void (MMV) approach. Comput Methods Appl Mech Eng 344:798–818. https://doi.org/10.1016/j.cma.2018.10.011
Tan XP, Tan YJ, Chow CSL, Tor SB, Yeong WY (2017) Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: a state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater Sci Eng C Mater Biol Appl 76:1328–1343. https://doi.org/10.1016/j.msec.2017.02.094
Li SJ, Xu QS, Wang Z, Hou WT, Hao YL, Yang R, Murr LE (2014) Influence of cell shape on mechanical properties of Ti–6Al–4V meshes fabricated by electron beam melting method. Acta Biomater 10(10):4537–4547. https://doi.org/10.1016/j.actbio.2014.06.010
Ataee A, Li Y, Fraser D, Song G, Wen CJM, Design (2018) Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater Des 137(jan.):345–354. https://doi.org/10.1016/j.matdes.2017.10.040
Arash A, Yuncang L, Milan B, Cuie WJAM (2018) Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications. Acta Metarialia 158:354–368. https://doi.org/10.1016/j.actamat.2018.08.005
S Zhao WTH, QS Xu, SJ Li, YL Hao, R Yang (2018) Ti-6Al-4V lattice structures fabricated by electron beam melting for biomedical applications. Titanium in Medical and Dental Applications:277-301
Tan X, Tan YJ 2018 3D printing of metallic cellular scaffolds for bone implants. p. 297-316
Docquier P-L, Paul L, Cartiaux O, Delloye C, Banse X (2010) Computer-assisted resection and reconstruction of pelvic tumor sarcoma. Sarcoma 2010:125162. https://doi.org/10.1155/2010/125162
Tack P, Victor J, Gemmel P, Annemans L (2016) 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 15(1):115. https://doi.org/10.1186/s12938-016-0236-4
Al-Tamimi AA, Huang B, Vyas C, Hernandez M, Peach C, Bartolo P (2019) Topology optimised metallic bone plates produced by electron beam melting: a mechanical and biological study. Int J Adv Manuf Technol 104:104–210. https://doi.org/10.1007/s00170-019-03866-0
Bobbert FSL, Lietaert K, Eftekhari AA, Pouran B, Ahmadi SM, Weinans H, Zadpoor AA (2017) Additively manufactured metallic porous biomaterials based on minimal surfaces: a unique combination of topological, mechanical, and mass transport properties. Acta Biomater 53:572–584. https://doi.org/10.1016/j.actbio.2017.02.024
Chen C-S, Cheng C-K, Liu C-L, Lo W-H (2001) Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Med Eng Phys 23(7):485–493. https://doi.org/10.1016/S1350-4533(01)00076-5
Al-Tamimi AA, Fernandes (2017) Metallic bone fixation implants: a novel design approach for reducing the stress shielding phenomenon. Virtual Phys Prototy 12(2):141–151. https://doi.org/10.1080/17452759.2017.1307769
WH HR, van Rietbergen B (1992) The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res 274:124–134
Liu J, Zhan Y, Tian F, Liu M, Zhao X (2019) Optimization of artificial bone internal structure by topology optimization: finite element analysis. IOP Conference Series: Materials Science and Engineering 493. https://doi.org/10.1088/1757-899x/493/1/012149
Zhong Z-C, Wei S-H, Wang J-P (2006) Finite element analysis of the lumbar spine with a new cage using a topology optimization method. Med Eng Phys 28(1):90–98. https://doi.org/10.1016/j.medengphy.2005.03.007
Augat P, von Rüden C (2018) Evolution of fracture treatment with bone plates. Injury 49:S2–S7. https://doi.org/10.1016/S0020-1383(18)30294-8
Prasad K, Bazaka O, Chua M, Rochford M, Fedrick L, Spoor J, Symes R, Tieppo M, Collins C, Cao A, Markwell D, Ostrikov KK, Bazaka K (2017) Metallic biomaterials: current challenges and opportunities. Materials (Basel) 10(8). https://doi.org/10.3390/ma10080884
Uhthoff HK, Poitras P, Backman DS (2006) Internal plate fixation of fractures: short history and recent developments. J Orthop Sci 11(2):118–126. https://doi.org/10.1007/s00776-005-0984-7
Al-Tamimi AA, P Chris, Bartolo Paulo (2018) Topology optimization of metallic locking compression plates produced using electron beam melting. Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (Pro-AM 2018). 10.25341/D41G66
Bruns TE (2005) A reevaluation of the SIMP method with filtering and an alternative formulation for solid–void topology optimization. STRUCT MULTIDISCIP O 30(6):428–436. https://doi.org/10.1007/s00158-005-0537-x
Rozvany GIN (2001) Aims, scope, methods, history and unified terminology of computer-aided topology optimization in structural mechanics. Struct Multidiscip Optim 21:90–108. https://doi.org/10.1007/s001580050174
Al-Tamimi AA, Quental C, Folgado J, Peach C, Bartolo P (2019) Stress analysis in a bone fracture fixed with topology-optimised plates. Biomech Model Mechanobiol 19:693–699. https://doi.org/10.1007/s10237-019-01240-3
Lovald S, Baack B, Gaball C, Gartb O (2010) Biomechanical optimization of bone plates used in rigid fixation of mandibular symphysis fractures. J Oral Maxillofac Surg 68(8):1833–1841. https://doi.org/10.1016/j.joms.2009.09.108
Teo JWC, Khan SF 2019 Topology optimization of mandible fracture plate. IOP Conference Series: Materials Science and Engineering: IOP Publishing; 2019. p. 012049
Liu Y, Fan Y, Jiang X (2017) A customized fixation plate with novel structure designed by topological optimization for mandibular angle fracture based on finite element analysis. Biomed Eng Online 16(1):131–142. https://doi.org/10.1186/s12938-017-0422-z
Ouyang H, Deng Y, Xie P, Yang Y, Jiang B, Zeng C, Huang W (2017) Biomechanical comparison of conventional and optimised locking plates for the fixation of intraarticular calcaneal fractures: a finite element analysis. COMPUT METHOD BIOMEC 20(12):1339–1349. https://doi.org/10.1080/10255842.2017.1361938
Şensoy AT, Kaymaz I, Ertaş Ü (2020) Development of particle swarm and topology optimization-based modeling for mandibular distractor plates. Swarm and Evolutionary Computation 53:53. https://doi.org/10.1016/j.swevo.2020.100645
Freutel M, Galbusera F, Ignatius A, Dürselen L (2015) Material properties of individual menisci and their attachments obtained through inverse FE-analysis. J Biomech 48(8):1343–1349. https://doi.org/10.1016/j.jbiomech.2015.03.014
Wu C, Zheng K, Fang J, Steven GP, Li Q (2020) Time-dependent topology optimization of bone plates considering bone remodeling. Comput Methods Appl Mech Eng:359. https://doi.org/10.1016/j.cma.2019.112702
Lemón L (2016) Topology optimization process for new designs of reconstruction plates used for bridging large mandibular defects. Department of Biomedical Engineering 34(15):534–539
Sutradhar A, Park J, Carrau D, M JM (2014) Experimental validation of 3D printed patient-specific implants using digital image correlation and finite element analysis. Comput Biol Med 52:8–17. https://doi.org/10.1016/j.compbiomed.2014.06.002
Sutradhar A, Park J, Carrau D (2016) Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med Biol Eng Comput 54(7):1123–1135. https://doi.org/10.1007/s11517-015-1418-0
Dai N, Zhu J-F, Zhang M, Meng L-Y, Yu X-L, Zhang Y-H, Liu B-Y, Zhang S-L (2018) Design of a maxillofacial prosthesis based on topology optimization. J Mech Med Biol 18(0):1850024. https://doi.org/10.1142/s0219519418500240
Luo D, Rong Q, Chen Q (2017) Finite-element design and optimization of a three-dimensional tetrahedral porous titanium scaffold for the reconstruction of mandibular defects. Med Eng Phys 47:176–183. https://doi.org/10.1016/j.medengphy.2017.06.015
CarlosA Gómez Pérez HIM-C 2017 Raquel Espinosa-Castañeda, editor computer assisted design and structural topology optimization of customized craniofacial implants. Proceedings of the ASME International Mechanical Engineering Congress and Exposition; 2017
Iqbal T, Wang L, Li D, Dong E, Fan H, Fu J, Hu C (2019) A general multi-objective topology optimization methodology developed for customized design of pelvic prostheses. Med Eng Phys 69:8–16. https://doi.org/10.1016/j.medengphy.2019.06.008
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. JBJS 89(4):780–785. https://doi.org/10.2106/JBJS.F.00222
Azzam K, Meneghini RM 2017 Periprosthetic fracture of the femur after total hip arthroplasty. complications after primary total hip arthroplasty. p. 105-17
Cooke PH, Newman JH (1988) Fractures of the femur in relation to cemented hip prostheses. The Journal of bone and joint surgery British volume 70(3):386–389
Ries C, Boese CK, Dietrich F, Miehlke W, Heisel C (2019) Femoral stem subsidence in cementless total hip arthroplasty: a retrospective single-centre study. Int Orthop 43(2):307–314. https://doi.org/10.1007/s00264-018-4020-x
Kang H, Lin C-Y, Hollister SJ (2010) Topology optimization of three dimensional tissue engineering scaffold architectures for prescribed bulk modulus and diffusivity. Struct Multidiscip O 42(4):633–644. https://doi.org/10.1007/s00158-010-0508-8
Fernandes P, Rodrigues H, Jacobs C (1999) A model of bone adaptation using a global optimisation criterion based on the trajectorial theory of Wolff. Comput Method Biomec 2(2):125–138. https://doi.org/10.1080/10255849908907982
Munteanu S, Munteanu D, Gheorghiu B, Bedo T, Gabor C, Cremascoli P, Alemani F, Pop MA (2019) Additively manufactured femoral stem topology optimization: case study. Materials Today: Proceedings 19:1019–1025. https://doi.org/10.1016/j.matpr.2019.08.016
Fraldi M, Esposito L, Perrella G (2010) Topological optimization in hip prosthesis design. Biomech Model Mechanobiol 9(4):389–402. https://doi.org/10.1007/s10237-009-0183-0
Kharmanda G (2016) Integration of multi-objective structural optimization into cementless hip prosthesis design: improved Austin-Moore model. Comput Method Biomec 19(14):1557–1566. https://doi.org/10.1080/10255842.2016.1170121
Mohammad Reza Niroomand FB 2018 editor application of topology optimization in design of stem profile in hip implants using finite element method. Int J Advanced Design and Manufacturing Technology
Rajaa S. Abass MAA, Musaddiq Al Ali 2019 editor shape and topology optimization design for total hip joint implant. Proceedings of the World Congress on Engineering; 2019; London, U.K
Xie YMSG (1993) A simple evolutionary procedure for structural optimization. Comput Struct 49(5):885–896. https://doi.org/10.1016/0045-7949(93)90035-C
Rahchamani R, Soheilifard R (2019) Three-dimensional structural optimization of a cementless hip stem using a bi-directional evolutionary method. Comput Methods Biomech Biomed Engin:1–11. https://doi.org/10.1080/10255842.2019.1661387
Boyle C, Kim IY (2011) Comparison of different hip prosthesis shapes considering micro-level bone remodeling and stress-shielding criteria using three-dimensional design space topology optimization. J Biomech 44(9):1722–1728. https://doi.org/10.1016/j.jbiomech.2011.03.038
Kim IY, Kwak BM (2002) Design space optimization using a numerical design continuation method. INT J NUMER METH ENG 53(8):1979–2002. https://doi.org/10.1002/nme.369
Andrade-Campos A, Ramos A, Simões JA (2012) A model of bone adaptation as a topology optimization process with contact. J Biomed Sci Eng 05(05):229–244. https://doi.org/10.4236/jbise.2012.55030
Kutyłowski R, Szwechłowicz M (2019) Application of topology optimization to thighbone and thighbone/implant structure modelling. ARCH CIV MECH ENG 19(4):1006–1019. https://doi.org/10.1016/j.acme.2019.05.007
Marinela Petoa ER-C, C Adriana Hernándezd, Hector R Siller, editor 2019 Structural design optimization of knee replacement implants for Additive Manufacturing. 47th SME North American Manufacturing Research Conference; Penn State Behrend Erie Pennsylvania
Katz JN (1995) Lumbar spinal fusion. Surgical rates, costs, and complications. Spine (Phila Pa 1976) 20(20):78S–83S
Eck KR, Bridwell KH, Ungacta FF, Lapp MA, Lenke LG, Riew KD (2000) Analysis of titanium mesh cages in adults with minimum two-year follow-up. Spine (Phila Pa 1976) 25(18):2407–2415
Lonstein JE (2000) Four-year follow-up results of lumbar spine arthrodesis using Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976) 25(20):1506–1508
Mcafee PC (1999) Interbody fusion cages in reconstructive operations on the spine. JBJS 81(6):859–880
ŽIVČÁK J, H R, SCHNITZER M, KULA T (2018) Numerical simulation and experimental testing of topologically optimized PLA cervical implants made by additive manufacturing methodics. acta mechanica et automatica 12:141–144. https://doi.org/10.2478/ama-2018-0022
Wang H, Wan Y, Liu X, Ren B, Liu Z, Zhang X, Yu M, editors. Macroscopic topology optimization of fusion cages used in TLIF Surgery2019; Singapore: Springer Singapore
Tovar A, Gano SE, Mason JJ (2005) Optimum design of an interbody implant for lumbar spine fixation. Adv Eng Softw 36(9):634–642. https://doi.org/10.1016/j.advengsoft.2005.03.008
Lin HM, Liu C, Pan Y (2014) Biomechanical analysis and design of a dynamic spinal fixator using topology optimization: a finite element analysis. Med Biol Eng Comput 52(5):499–508. https://doi.org/10.1007/s11517-014-1154-x
Chen CS, Shih SL (2018) Biomechanical analysis of a new lumbar interspinous device with optimized topology. Med Biol Eng Comput 56(8):1333–1341. https://doi.org/10.1007/s11517-017-1767-y
Guo LX, Yin JY (2019) Finite element analysis and design of an interspinous device using topology optimization. Med Biol Eng Comput 57(1):89–98. https://doi.org/10.1007/s11517-018-1838-8
Chang CL, Chen CS, Huang C (2012) Finite element analysis of the dental implant using a topology optimization method. Med Eng Phys 34(7):999–1008. https://doi.org/10.1016/j.medengphy.2012.06.004
Zhongpu Z, Junning C, Eric L, Wei L, Michael S, Qing L (2016) Topological design of all-ceramic dental bridges for enhancing fracture resistance. INT J NUMER METH BIO 32(6):e02749. https://doi.org/10.1002/cnm.2749
Seitz KF, Grabe J, Kohne T (2018) A three-dimensional topology optimization model for tooth-root morphology. Comput Methods Biomech Biomed Engin 21(2):177–185. https://doi.org/10.1080/10255842.2018.1431778
Polgar K, Gill HS, Viceconti M, Murray DW, O'Connor JJ (2003) Strain distribution within the human femur due to physiological and simplified loading: finite element analysis using the muscle standardized femur model. Proc Inst Mech Eng H J Eng Med 217(3):173–189. https://doi.org/10.1243/095441103765212677
Čapek J, Machová M, Fousová M, Kubásek J, Vojtěch D, Fojt J, Jablonská E, Lipov J, Ruml T (2016) Highly porous, low elastic modulus 316 L stainless steel scaffold prepared by selective laser melting. Mater Sci Eng C 69:631–639. https://doi.org/10.1016/j.msec.2016.07.027
Bruder SP, Kraus KH, Goldberg VM, Kadiyala S (1998) The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. JBJS 80(7):985–996
Cheah C-M, Chua C-K, Leong K-F, Cheong C-H, Naing M-W (2004) Automatic algorithm for generating complex polyhedral scaffold structures for tissue engineering. Tissue Eng 10(3-4):595–610. https://doi.org/10.1089/107632704323061951
Dias MR, Guedes JM, Flanagan CL, Hollister SJ, Fernandes PR (2014) Optimization of scaffold design for bone tissue engineering: a computational and experimental study. Med Eng Phys 36(4):448–457. https://doi.org/10.1016/j.medengphy.2014.02.010
Fang Z, Starly B, Sun W (2005) Computer-aided characterization for effective mechanical properties of porous tissue scaffolds. COMPUT AIDED DESIGN 37(1):65–72. https://doi.org/10.1016/j.cad.2004.04.002
Giannitelli SM, Accoto D, Trombetta M, Rainer A (2014) Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater 10(2):580–594. https://doi.org/10.1016/j.actbio.2013.10.024
Hollister SJ (2002) An image-based approach fordesigning and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg 29:67–71. https://doi.org/10.1034/j.1399-0020.2000.290115.x
Hollister SJ, Maddox RD, Taboas JM (2002) Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 23(20):4095–4103. https://doi.org/10.1016/S0142-9612(02)00148-5
Sun W, Starly B, Darling A, Gomez C (2004) Computer-aided tissue engineering: application to biomimetic modelling and design of tissue scaffolds. BIOTECHNOL Appl Bioc 39(1):49–58. https://doi.org/10.1042/BA20030109
Gogarty E, Pasini D. Hierarchical topology optimization for bone tissue scaffold: preliminary results on the design of a fracture fixation plate2015. 311-40 p
Sigmund O (1994) Materials with prescribed constitutive parameters: an inverse homogenization problem. Int J Solids Struct 31(17):2313–2329. https://doi.org/10.1016/0020-7683(94)90154-6
Jia D, Li F, Zhang C, Liu K, Zhang Y (2019) Design and simulation analysis of lattice bone plate based on finite element method. Mech Adv Mater Struct:1–11. https://doi.org/10.1080/15376494.2019.1665759
Cheng K, Liu Y, Yao C, Zhao W, Xu X (2019) A personalized mandibular implant with supporting and porous structures designed with topology optimization – a case study of canine. Rapid Prototyp J 25(2):417–426. https://doi.org/10.1108/rpj-11-2017-0231
Haden CV, Collins PC, Harlow DGJJ (2015) Yield strength prediction of titanium alloys. JOM 67(6):1357–1361. https://doi.org/10.1007/s11837-015-1436-2
Hu J, Wang JH, Wang R, Yu XB, Liu Y, Baur DA (2019) Analysis of biomechanical behavior of 3D printed mandibular graft with porous scaffold structure designed by topological optimization. 3D Print Med 5(1):5. https://doi.org/10.1186/s41205-019-0042-2
Arabnejad Khanoki S, Pasini D (2012) Multiscale design and multiobjective optimization of orthopedic hip implants with functionally graded cellular material. J Biomech Eng 134(3):031004. https://doi.org/10.1115/1.4006115
Yuhao He DB, David Durocher, James M. Gilbert, editor 2018 Solid-lattice hip prosthesis design: applying topology optimization to reduce stress shielding from hip implants. Proceedings of the Design of Medical Devices Conference; 2018
Ying Lin C-C, Chen Q (2004) Interbody fusion cage design using integrated global layout and local microstructure topology optimization. Spine (Phila Pa 1976) 29:1747–1754. https://doi.org/10.1097/01.BRS.0000134573.14150.1A
Lin C. Scaffold internal architecture design for porosity and elastic properties by a topology microstructure optimization method. Annual Meeting of Society for Biomaterials Reno, Nevada, May 2, 2003; 20032003
Xu Y, Zhang D, Zhou Y, Wang W, Cao X (2017) Study on topology optimization design, manufacturability, and performance evaluation of Ti-6Al-4V porous structures fabricated by selective laser melting (SLM). Materials (Basel) 10(9). https://doi.org/10.3390/ma10091048
Hollister S (2009) Scaffold design and manufacturing: from concept to clinic. Adv Mater Weinheim 21(32-33):3330–3342. https://doi.org/10.1002/adma.200802977
Lin CY, Kikuchi N, Hollister SJ (2004) A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J Biomech 37(5):623–636. https://doi.org/10.1016/j.jbiomech.2003.09.029
Lin CY, Wirtz T, LaMarca F, Hollister SJ (2007) Structural and mechanical evaluations of a topology optimized titanium interbody fusion cage fabricated by selective laser melting process. J Biomed Mater Res A 83(2):272–279. https://doi.org/10.1002/jbm.a.31231
CL SJH, Saito E (2005) Engineering craniofacial scaffolds. ORTHOD CRANIOFAC RES 8:162–173. https://doi.org/10.1111/j.1601-6343.2005.00329.x
Fernandes P, Guedes JM, Rodrigues H (1999) Topology optimization of three-dimensional linear elastic structures with a constraint on “perimeter”. Comput Struct 73(6):583–594. https://doi.org/10.1016/S0045-7949(98)00312-5
Sigmund O (1994) Design of material structures using topology optimization
Kang H, Hollister SJ, La Marca F (2013) Porous biodegradable lumbar interbody fusion cage design and fabrication using integrated global-local topology optimization with laser sintering. J Biomech Eng 135(10):101013–101018. https://doi.org/10.1115/1.4025102
Ying L, Chengyu L, Scott JH (2013) A new approach for designing biodegradable bone tissue augmentation devices by using degradation topology optimization. Journal of Systemics Cybernetics & Informatics 11
Braune S, Lendlein A, Jung F 2018 3 - Developing standards and test protocols for testing the hemocompatibility of biomaterials A2 - Siedlecki, Christopher A. Hemocompatibility of Biomaterials for Clinical Applications: Woodhead Publishing. p. 51-76
Knutsen AR, Borkowski SL, Ebramzadeh E, Flanagan CL, Hollister SJ, Sangiorgio SN (2015) Static and dynamic fatigue behavior of topology designed and conventional 3D printed bioresorbable PCL cervical interbody fusion devices. J Mech Behav Biomed Mater 49:332–342. https://doi.org/10.1016/j.jmbbm.2015.05.015
Liu YJ, Ren DC, Li SJ, Wang H, Zhang LC, Sercombe TB (2020) Enhanced fatigue characteristics of a topology-optimized porous titanium structure produced by selective laser melting. Additive Manufacturing 32:101060. https://doi.org/10.1016/j.addma.2020.101060
Ahsan AMMN, Xie R, Khoda B, editors 2017 Direct bio-printing with heterogeneous topology design. 45th SME North American Manufacturing Research Conference
Kang H, Long JP, Urbiel Goldner GD, Goldstein SA, Hollister SJ (2012) A paradigm for the development and evaluation of novel implant topologies for bone fixation: implant design and fabrication. J Biomech 45(13):2241–2247. https://doi.org/10.1016/j.jbiomech.2012.06.011
Funding
This work was supported by (1) the National Natural Science Foundation of China (grant numbers 82072456&81802174), (2) the National Key R&D Program of China (No. 2018YFB1105100), (3) the Department of Science and Technology of Jilin Province, P.R.C. (grant numbers 20200404202YY&20200403086SF&20200201453JC), (4) the Department of finance in Jilin province (grant number2019SCZT046&2020SCZT037&201817294302), (5) the Undergraduate teaching reform research project of Jilin University (grant number 4Z2000610852), (6) the Key training plan for outstanding young teachers of Jilin University (grant number 419080520253), (7) the Bethune plan of Jilin University (grant number 470110000692), and (8) the Jilin Province Development and Reform Commission, P.R.C. (grant numbers 2018C010).
Author information
Authors and Affiliations
Contributions
The idea was come up with Jincheng Wang and Qing Han. The first draft of the manuscript was written by Naichao Wu. The literature search was performed by Shan Li and Boyan Zhang. This review was revised by Qing Han, Chenyu Wang, and Bingpeng Chen. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, N., Li, S., Zhang, B. et al. The advances of topology optimization techniques in orthopedic implants: A review. Med Biol Eng Comput 59, 1673–1689 (2021). https://doi.org/10.1007/s11517-021-02361-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02361-7