Abstract
Segmentation of intracerebral hemorrhage (ICH) helps improve the quality of diagnosis, draft the desired treatment methods, and clinically observe the variations with healthy patients. The clinical utilization of various ICH progression scoring systems has limitations due to the systems’ modest predictive value. This paper proposes a single pipeline of a multi-task model for end-to-end hemorrhage segmentation and risk estimation. We introduce a 3D spatial attention unit and integrate it into the state-of-the-art segmentation architecture, UNet, to enhance the accuracy by bootstrapping the global spatial representation. We further extract the geometric features from the segmented hemorrhage volume and fuse them with clinical features such as CT angiography (CTA) spot, Glasgow Coma Scale (GCS), and age to predict the ICH stability. Several state-of-the-art machine learning techniques such as multilayer perceptron (MLP), support vector machine (SVM), gradient boosting, and random forests are applied to train stability estimation and to compare the performances. To align clinical intuition with model learning, we determine the shapely values (SHAP) and explain the most significant features for the ICH risk scoring system. A total of 79 patients are included, of which 20 are found in critical condition. Our proposed single pipeline model achieves a segmentation accuracy of 86.3%, stability prediction accuracy of 78.3%, and precision of 82.9%; the mean square error of exact expansion rate regression is observed to be 0.46. The SHAP analysis reveals that CTA spot sign, age, solidity, location, and length of the first axis of the ICH volume are the most critical characteristics that help define the stability of the stroke lesion. We also show that integrating significant geometric features with clinical features can improve the ICH progression scoring by predicting long-term outcomes.

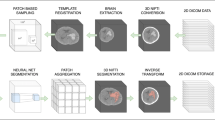
Overview of our proposed method comprising of spatial attention and feature extraction mechanisms. The architecture is trained on the input CT images, and the first step output is the predicted segmentation of the hemorrhagic region. The output is fed into a geometric feature extractor and is fused with clinical features to estimate ICH stability using a multilayer perceptron (MLP)









Similar content being viewed by others
References
Abouzari M, Rashidi A, Zandi-Toghani M, Behzadi M, Asadollahi M (2009) Chronic subdural hematoma outcome prediction using logistic regression and an artificial neural network. Neurosurg Rev 32(4):479–484
Arora T, Dhir R (2017) Correlation-based feature selection and classification via regression of segmented chromosomes using geometric features. Med Biol Eng Comput 55(5):733–745
Bakas S, Reyes M, Jakab A, Bauer S, Rempfler M, Crimi A, Shinohara RT, Berger C, Ha SM, Rozycki M et al (2018) Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the brats challenge. arXiv:181102629
Bentley P, Ganesalingam J, Jones A L C, Mahady K, Epton S, Rinne P, Sharma P, Halse O, Mehta A, Rueckert D (2014) Prediction of stroke thrombolysis outcome using ct brain machine learning. NeuroImage: Clinical 4:635–640
Blacquiere D, Demchuk A M, Al-Hazzaa M, Deshpande A, Petrcich W, Aviv R I, Rodriguez-Luna D, Molina C A, Silva Blas Y, Dzialowski I et al (2015) Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke 46(11):3111–3116
Boulouis G, Morotti A, Brouwers H B, Charidimou A, Jessel M J, Auriel E, Pontes-Neto O, Ayres A, Vashkevich A, Schwab K M et al (2016) Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol 73 (8):961–968
Brouwers H B, Greenberg S M (2013) Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis 35(3):195–201
Brügger R, Baumgartner CF, Konukoglu E (2019) A partially reversible u-net for memory-efficient volumetric image segmentation. arXiv:190606148
Chen S, Zhao B, Wang W, Shi L, Reis C, Zhang J (2017) Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget 8(51):89348
Cheung R T F, Zou L Y (2003) Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 34(7):1717–1722
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3d u-net: learning dense volumetric segmentation from sparse annotation. In: International conference on medical image computing and computer-assisted intervention. Springer, pp 424–432
Demchuk A M, Dowlatshahi D, Rodriguez-Luna D, Molina C A, Blas Y S, Dzialowski I, Kobayashi A, Boulanger J M, Lum C, Gubitz G et al (2012) Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the ct-angiography spot sign (predict): a prospective observational study. Lancet Neurol 11(4):307–314
Hariharan B, Arbeláez P, Girshick R, Malik J (2015) Hypercolumns for object segmentation and fine-grained localization. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 447–456
Hesamian M H, Jia W, He X, Kennedy P (2019) Deep learning techniques for medical image segmentation: achievements and challenges. J Digit Imaging 32(4):582–596
Hssayeni M D, Croock M S, Salman A D, Al-khafaji H F, Yahya Z A, Ghoraani B (2020) Intracranial hemorrhage segmentation using a deep convolutional model. Data 5(1):14
Hu J, Shen L, Sun G (2018) Squeeze-and-excitation networks. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 7132–7141
Ibrahim G M, Morgan B R, Macdonald R L (2014) Patient phenotypes associated with outcomes after aneurysmal subarachnoid hemorrhage: a principal component analysis. Stroke 45(3):670–676
Islam M, Sanghani P, See AAQ, James ML, King NKK, Ren H (2018) Ichnet: Intracerebral hemorrhage (ICH) segmentation using deep learning. In: International MICCAI Brainlesion Workshop. Springer, pp 456–463
Islam M, Vaidyanathan NR, Jose VJM, Ren H (2019) Ischemic stroke lesion segmentation using adversarial learning. In: Crimi A, Bakas S, Kuijf H, Keyvan F, Reyes M, van Walsum T (eds) Brainlesion: Glioma, multiple sclerosis, stroke and traumatic brain injuries. https://doi.org/10.1007/978-3-030-11723-8_29. Springer International Publishing, Cham, pp 292–300
Islam M, Wijethilake N, Ren H (2021) Glioblastoma multiforme prognosis: Mri missing modality generation, segmentation and radiogenomic survival prediction. Computerized Medical Imaging and Graphics: 101906
Kingma DP, Ba J (2014) Adam: A method for stochastic optimization. arXiv:14126980
Lee K, Zung J, Li P, Jain V, Seung HS (2017) Superhuman accuracy on the snemi3d connectomics challenge. arXiv:170600120
Lehtinen J, Munkberg J, Hasselgren J, Laine S, Karras T, Aittala M, Aila T (2018) Noise2noise: Learning image restoration without clean data. arXiv:180304189
Lei T, Wang R, Wan Y, Du X, Meng H, Nandi AK (2020) Medical image segmentation using deep learning: A survey. arXiv:200913120
Li Q, Huang Y J, Zhang G, Lv F J, Wei X, Dong M X, Chen J J, Zhang L J, Qin X Y, Xie P (2015a) Intraventricular hemorrhage and early hematoma expansion in patients with intracerebral hemorrhage. Sci Reports 5:11357
Li Q, Zhang G, Huang Y J, Dong M X, Lv F J, Wei X, Chen J J, Zhang L J, Qin X Y, Xie P (2015b) Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke 46(8):2119–2123
Liaqat A, Khan M A, Shah J H, Sharif M, Yasmin M, Fernandes S L (2018) Automated ulcer and bleeding classification from wce images using multiple features fusion and selection. J Mech Med Biol 18(04):1850038
Litjens G, Kooi T, Bejnordi B E, Setio A A A, Ciompi F, Ghafoorian M, Van Der Laak J A, Van Ginneken B, Sánchez C I (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88
Lundberg SM, Lee SI (2017) A unified approach to interpreting model predictions. In: Advances in neural information processing systems, pp 4765–4774
Maas M B, Nemeth A J, Rosenberg N F, Kosteva A R, Prabhakaran S, Naidech A M (2013) Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology 80(14):1295–1299
Mardia K V (1970) Measures of multivariate skewness and kurtosis with applications. Biometrika 57(3):519–530
Murtagh F (1991) Multilayer perceptrons for classification and regression. Neurocomputing 2 (5-6):183–197
Oktay O, Schlemper J, Folgoc LL, Lee M, Heinrich M, Misawa K, Mori K, McDonagh S, Hammerla NY, Kainz B, et al. (2018) Attention u-net: Learning where to look for the pancreas. arXiv:180403999
Peng W J, Reis C, Reis H, Zhang J, Yang J (2017) Predictive value of cta spot sign on hematoma expansion in intracerebral hemorrhage patients. BioMed research international 2017
Poli L, Leuci E, Costa P, De Giuli V, Caria F, Candeloro E, Persico A, Gamba M, Magoni M, Micieli G et al (2019) Validation and comparison of noncontrast ct scores to predict intracerebral hemorrhage expansion. Neurocritical Care 32:804–811
Burchell SR, Tang J, Zhang JH (2017) Hematoma expansion following intracerebral hemorrhage: mechanisms targeting the coagulation cascade and platelet activation. Current Drug Targets 18(12):1329–1344
Ren H, Dupont P E (2012) Tubular enhanced geodesic active contours for continuum robot detection using 3d ultrasound. In: ICRA2012, IEEE International conference on robotics and automation, 14–18 May, St. Paul, pp 2907–2912. https://doi.org/10.1109/ICRA.2012.6225033
Ronneberger O, Fischer P, Brox T (2015) U-net: Convolutional networks for biomedical image segmentation. In: International conference on medical image computing and computer-assisted intervention. MICCAI 2015, Part III, LNCS 9351. Springer, pp 234–241. https://doi.org/10.1007/978-3-319-24574-4_28
Roy A G, Navab N, Wachinger C (2018) Recalibrating fully convolutional networks with spatial and channel “squeeze and excitation” blocks. IEEE Trans Med Imaging 38(2):540–549
Sanghani P, Ti A B, King N K K, Ren H (2018) Overall survival prediction in glioblastoma multiforme patients from volumetric, shape and texture features using machine learning. Surg Oncol 27(4):709–714. https://doi.org/10.1016/j.suronc.2018.09.002
Sanghani P, Ang B T, King N K K, Ren H (2019a) Regression based overall survival prediction of glioblastoma multiforme patients using a single discovery cohort of multi-institutional multi-channel mr images. Med Biol Eng Comput 57(8):1683–1691. https://doi.org/10.1007/s11517-019-01986-z
Sanghani P, Ti AB, King NKK, Ren H (2019b) Evaluation of tumor shape features for overall survival prognosis in glioblastoma multiforme patients. Surgical Oncology 29:178–183. https://doi.org/10.1016/j.suronc.2019.05.005, http://www.sciencedirect.com/science/article/pii/S0960740419300829
Schlemper J, Oktay O, Schaap M, Heinrich M, Kainz B, Glocker B, Rueckert D (2019) Attention gated networks: Learning to leverage salient regions in medical images. Med Image Anal 53:197–207
Sengar N, Dutta MK, Burget R, Ranjoha M (2017) Automated detection of suspected glaucoma in digital fundus images. In: 2017 40th International Conference on Telecommunications and Signal Processing (TSP), pp 749–752. https://doi.org/10.1109/TSP.2017.8076088
Sharif M, Attique Khan M, Rashid M, Yasmin M, Afza F, Tanik U J (2019) Deep cnn and geometric features-based gastrointestinal tract diseases detection and classification from wireless capsule endoscopy images. J Exp Theor Artif Intell, pp 577–599
Smith S M (2002) Fast robust automated brain extraction. Human Brain Mapping 17(3):143–155
Wada R, Aviv R I, Fox A J, Sahlas D J, Gladstone D J, Tomlinson G, Symons S P (2007) Ct angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38 (4):1257–1262
Wang C W, Liu Y J, Lee Y H, Hueng D Y, Fan H C, Yang F C, Hsueh C J, Kao H W, Juan C J, Hsu H H (2014) Hematoma shape, hematoma size, glasgow coma scale score and ich score: which predicts the 30-day mortality better for intracerebral hematoma? PloS one 9(7):e102326
Winzeck S, Hakim A, McKinley R, Pinto JAADSR, Alves V, Silva C, Pisov M, Krivov E, Belyaev M, Monteiro M, Oliveira A, Choi Y, Paik MC, Kwon Y, Lee H, Kim BJ, Won JH, Islam M, Ren H, Robben D, Suetens P, Gong E, Niu Y, Xu J, Pauly JM, Lucas C, Heinrich MP, Rivera LC, Castillo LS, Daza LA, Beers AL, Arbelaezs P, Maier O, Chang K, Brown JM, Kalpathy-Cramer J, Zaharchuk G, Wiest R, Reyes M (2018) Isles 2016 and 2017-benchmarking ischemic stroke lesion outcome prediction based on multispectral mri. Front Neurol 9:679. https://doi.org/10.3389/fneur.2018.00679. https://www.frontiersin.org/article/10.3389/fneur.2018.00679
Wu D, Liu D, Suehling M, Tietjen C, Soza G, Zhou KS (2012) Automatic detection of liver lesion from 3d computed tomography images. In: 2012 IEEE Computer society conference on computer vision and pattern recognition workshops, pp 31–37 https://doi.org/10.1109/CVPRW.2012.6239244
Funding
This work is supported by the Singapore Academic Research Fund under Grant R397000353114 and the Shun Hing Institute of Advanced Engineering (SHIAE project BME-p1-21, 8115064) at the Chinese University of Hong Kong (CUHK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rangaraj, S., Islam, M., VS, V. et al. Identifying risk factors of intracerebral hemorrhage stability using explainable attention model. Med Biol Eng Comput 60, 337–348 (2022). https://doi.org/10.1007/s11517-021-02459-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02459-y