Abstract
Objective
Software-based devices have increasingly become an important part of several clinical scenarios. Due to their critical impact on human life, medical devices have very strict safety requirements. It is therefore necessary to apply verification methods to ensure that the safety requirements are met. Verification of software-based devices is commonly limited to the verification of their internal elements without considering the interaction that these elements have with other devices as well as the application environment in which they are used. Medical guidelines define clinical procedures, which contain the necessary information to completely verify medical devices. The objective of this work was to incorporate medical guidelines into the verification process in order to increase the reliability of the software-based medical devices.
Materials and methods
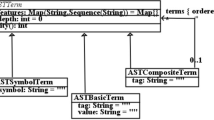
Medical devices are developed using the model-driven method deterministic models for signal processing of embedded systems (DMOSES). This method uses unified modeling language (UML) models as a basis for the development of medical devices. The UML activity diagram is used to describe medical guidelines as workflows. The functionality of the medical devices is abstracted as a set of actions that is modeled within these workflows. In this paper, the UML models are verified using the UPPAAL model-checker. For this purpose, a formalization approach for the UML models using timed automaton (TA) is presented.
Results
A set of requirements is verified by the proposed approach for the navigation-guided biopsy. This shows the capability for identifying errors or optimization points both in the workflow and in the system design of the navigation device. In addition to the above, an open source eclipse plug-in was developed for the automated transformation of UML models into TA models that are automatically verified using UPPAAL.
Conclusions
The proposed method enables developers to model medical devices and their clinical environment using clinical workflows as one UML diagram. Additionally, the system design can be formally verified automatically.







Similar content being viewed by others
References
Health IT and Patient Safety (2012) Building safer systems for better care. Committee on patient safety and health information technology, Institute of Medicine
Lee EA (2006) The problem with threads. Technical report, EECS Department, University of California, Berkeley
Baier C, Katoen JP (2008) Principles of model checking (representation and mind series). MIT Press, Cambridge, MA
Pazzi L, Pradelli M (2008) A state-based systemic view of behavior for safe medical computer applications. In: Proceedings of the 2008 21st IEEE international symposium on computer-based medical systems, CBMS ’08. IEEE Computer Society, Washington, DC, USA, pp 108–113. doi:10.1109/CBMS.2008.94
Pérez B, Porres I (2008) Verification of clinical guidelines by model checking. In: Proceedings of the 2008 21st IEEE international symposium on computer-based medical systems, CBMS ’08. IEEE Computer Society, Washington, DC, USA, pp 114–119. doi:10.1109/CBMS.2008.86
Holzmann CJ (1997) The model checker SPIN. IEEE Trans Softw Eng 23:279–295
Maruster L, Jorna RJ (2005) From data to knowledge: a method for modeling hospital logistic processes. IEEE Trans Inf Technol Biomed 9(2):248–255. http://dblp.uni-trier.de/db/journals/titb/titb9.html/MarusterJ05
Hoare CAR (1983) Communicating sequential processes. Commun ACM 26:100–106
Faber J (2012) A timed model for healthcare workflows based on csp. In: Breu R, Hatcliff J (eds) SEHC 2012. IEEE, Zurich. doi:10.1109/SEHC.2012.6227002
Systems F (2012) Failure divergence refinement (fdr). http://www.fsel.com/
Pajic M, Mangharam R, Sokolsky O, David Arney JMG, Lee I (2011) IEEE transactions of industrial informatics (TII), Special section on cyber-physical systems (submitted)
Behrmann G, David R, Larsen KG (2004) A tutorial on UPPAAL. Springer, Berlin, pp 200–236. http://www.uppaal.org/
Daw Z, Vetter M (2009) Deterministic UML models with interconnected activities and state machines for embedded systems. In: Model driven engineering languages and systems, 12th international conference, MODELS 2009
Daw Z, Englert C, Alvarez F, Boercsoek J, Vetter M (2011) Model-driven timing analysis and verification for safety-critical embedded systems. In: Proceedings of the 24th international congress on condition monitoring and diagnostics, engineering management
Alur R, Dill DL (1994) A theory of timed automata. Theor Comput Sci 126(2):183–235
Emerson EA, Sistla AP (1984) Deciding branching time logic. In: Proceedings of the sixteenth annual ACM symposium on theory of computing, STOC ’84. ACM, New York, NY, USA, pp 14–24. doi:10.1145/800057.808661
Acknowledgments
This research is supported by the European Union, European Regional Development Fund (ERDF) and the federal state of Baden-Wuerttemberg, Germany.
Conflict of Interest
Zamira Daw, Rance Cleaveland, and Marcus Vetter declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daw, Z., Cleaveland, R. & Vetter, M. Formal verification of software-based medical devices considering medical guidelines. Int J CARS 9, 145–153 (2014). https://doi.org/10.1007/s11548-013-0919-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-013-0919-2