Abstract
Background
MR spectroscopy (MRS) measurements are common practice in the preoperative diagnostic regimen, but no evidence exists concerning their value in intraoperative MRI (iMRI) setting. We sought to examine the feasibility of intraoperative MRS and to assess the clinical value of the method in optimizing the gliomas resection.
Methods
Forty-five patients with low- and high-grade gliomas underwent iMRI-assisted surgery, including pre- and intraoperative MRS measurements. During the intraoperative control scan, MRS was performed at the resection margin. Peak areas under the major metabolites (N-acetyl-aspartate: NAA; choline: Cho; and creatine: Cr) resonances were estimated, and their ratios entered in the statistical analysis.
Results
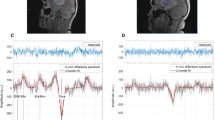
Concerning preoperative MRS imaging, mean Cho/NAA and Cho/Cr ratios in low-grade gliomas were 2.3 and 1.2, respectively. The average Cho/NAA and Cho/Cr ratios in the high-grade gliomas were 3.9 and 2.3, respectively. In 12 out of 20 cases with low-grade gliomas, intraoperative conventional MR imaging showed suspected tumor remnant and MRS diagnosed correctly the tissue signal alterations in 10 out of those 12 cases. MRS could characterize gadolinium-enhancing or non-enhancing tumor remnants in all cases with high-grade tumors. Thus, it could help achieve total tumor resection unless the latter was contraindicated due to increased risk of neurological complications.
Conclusions
MR spectroscopy (MRS) in an iMRI setting is feasible, facilitating preoperative glioma staging as well as satisfactory characterization of suspected tumor remnants. Thus, it may be helpful tool for an extended tumor resection.



Similar content being viewed by others
References
Gasser TG, Sandalcioglu EI, Wiedemayer H, Hans V, Gizewski E, Forsting M, Stolke D (2004) A novel passive functional MRI paradigm for preoperative identification of the somatosensory cortex. Neurosurg Rev 27(2):106–112. doi:10.1007/s10143-003-0318-1
Gasser T, Ganslandt O, Sandalcioglu E, Stolke D, Fahlbusch R, Nimsky C (2005) Intraoperative functional MRI: implementation and preliminary experience. Neuroimage 26(3):685–693. doi:10.1016/j.neuroimage.2005.02.022
Sun GC, Chen XL, Zhao Y, Wang F, Hou BK, Wang YB, Song ZJ, Wang D, Xu BN (2011) Intraoperative high-field magnetic resonance imaging combined with fiber tract neuronavigation-guided resection of cerebral lesions involving optic radiation. Neurosurgery 69(5):1070–1084; discussion 1084. doi:10.1227/NEU.0b013e3182274841
Nimsky C, Ganslandt O, Merhof D, Sorensen AG, Fahlbusch R (2006) Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage 30(4):1219–1229. doi:10.1016/j.neuroimage.2005.11.001
Roder C, Bender B, Ritz R, Honegger J, Feigl G, Naegele T, Tatagiba MS, Ernemann U, Bisdas S (2013) Intraoperative visualization of residual tumor: the role of perfusion-weighted imaging in a high-field intraoperative magnetic resonance scanner. Neurosurgery 72 (2 Suppl Operative):ons151–ons158; discussion ons158. doi:10.1227/NEU.0b013e318277c606
Keles GE (2004) Intracranial neuronavigation with intraoperative magnetic resonance imaging. Curr Opin Neurol 17(4):497–500
Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R (2006) Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res 28(5):482–487. doi:10.1179/016164106X115125
Kuhnt D, Ganslandt O, Schlaffer SM, Buchfelder M, Nimsky C (2011) Quantification of glioma removal by intraoperative high-field magnetic resonance imaging: an update. Neurosurgery 69(4):852–862; discussion 862–853. doi:10.1227/NEU.0b013e318225ea6b
Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, Jackson E, Sawaya R (2009) Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery 64(6):1073–1081; discussion 1081. doi:10.1227/01.NEU.0000345647.58219.07
Nakajima T, Kumabe T, Kanamori M, Saito R, Tashiro M, Watanabe M, Tominaga T (2009) Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurologia medico-chirurgica 49(9):394–401
Tedeschi G, Lundbom N, Raman R, Bonavita S, Duyn JH, Alger JR, Di Chiro G (1997) Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 87(4):516–524. doi:10.3171/jns.1997.87.4.0516
Peet AC, Arvanitis TN, Leach MO, Waldman AD (2012) Functional imaging in adult and paediatric brain tumours. Nat Rev Clin Oncol 9(12):700–711. doi:10.1038/nrclinonc.2012.187
Kreis R (2004) Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed 17(6):361–381. doi:10.1002/nbm.891
Garcia-Gomez JM, Luts J, Julia-Sape M, Krooshof P, Tortajada S, Robledo JV, Melssen W, Fuster-Garcia E, Olier I, Postma G, Monleon D, Moreno-Torres A, Pujol J, Candiota AP, Martinez-Bisbal MC, Suykens J, Buydens L, Celda B, Van Huffel S, Arus C, Robles M (2009) Multiproject-multicenter evaluation of automatic brain tumor classification by magnetic resonance spectroscopy. MAGMA 22(1):5–18. doi:10.1007/s10334-008-0146-y
Chen X, Xu BN, Meng X, Zhang J, Yu X, Zhou D (2012) Dual-room 1.5-T intraoperative magnetic resonance imaging suite with a movable magnet: implementation and preliminary experience. Neurosurg Rev 35(1):95–109; discussion 109–110. doi:10.1007/s10143-011-0336-3.
Pamir MN, Ozduman K, Dincer A, Yildiz E, Peker S, Ozek MM (2010) First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg 112(1):57–69. doi:10.3171/2009.3.JNS081139
Utriainen M, Komu M, Vuorinen V, Lehikoinen P, Sonninen P, Kurki T, Utriainen T, Roivainen A, Kalimo H, Minn H (2003) Evaluation of brain tumor metabolism with [11C]choline PET and 1H-MRS. J Neurooncol 62(3):329–338
Fulham MJ, Bizzi A, Dietz MJ, Shih HH, Raman R, Sobering GS, Frank JA, Dwyer AJ, Alger JR, Di Chiro G (1992) Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology 185(3):675– 686
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24(10):1989–1998
Pirzkall A, Nelson SJ, McKnight TR, Takahashi MM, Li X, Graves EE, Verhey LJ, Wara WW, Larson DA, Sneed PK (2002) Metabolic imaging of low-grade gliomas with three-dimensional magnetic resonance spectroscopy. Int J Radiat Oncol Biol Phys 53(5):1254–1264
Li X, Lu Y, Pirzkall A, McKnight T, Nelson SJ (2002) Analysis of the spatial characteristics of metabolic abnormalities in newly diagnosed glioma patients. J Magn Reson Imaging 16(3):229–237. doi:10.1002/jmri.10147
Senft C, Hattingen E, Pilatus U, Franz K, Schanzer A, Lanfermann H, Seifert V, Gasser T (2009) Diagnostic value of proton magnetic resonance spectroscopy in the noninvasive grading of solid gliomas: comparison of maximum and mean choline values. Neurosurgery 65(5):908–913; discussion 913. doi:10.1227/01.NEU.0000356982.82378.BA
Porto L, Kieslich M, Franz K, Lehrnbecher T, Zanella F, Pilatus U, Hattingen E (2011) MR spectroscopy differentiation between high and low grade astrocytomas: a comparison between paediatric and adult tumours. Eur J Paediatr Neurol 15(3):214–221. doi:10.1016/j.ejpn.2010.11.003
Kinoshita Y, Yokota A (1997) Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed 10(1):2–12. doi:10.1002/(SICI)1099-1492(199701)10:1<2:AID-NBM442>3.0.CO;2-N
Galanaud D, Chinot O, Nicoli F, Confort-Gouny S, Le Fur Y, Barrie-Attarian M, Ranjeva JP, Fuentes S, Viout P, Figarella-Branger D, Cozzone PJ (2003) Use of proton magnetic resonance spectroscopy of the brain to differentiate gliomatosis cerebri from low-grade glioma. J Neurosurg 98(2):269–276. doi:10.3171/jns.2003.98.2.0269
Hattingen E, Raab P, Franz K, Lanfermann H, Setzer M, Gerlach R, Zanella FE, Pilatus U (2008) Prognostic value of choline and creatine in WHO grade II gliomas. Neuroradiology 50(9):759–767. doi:10.1007/s00234-008-0409-3
Catalaa I, Henry R, Dillon WP, Graves EE, McKnight TR, Lu Y, Vigneron DB, Nelson SJ (2006) Perfusion, diffusion and spectroscopy values in newly diagnosed cerebral gliomas. NMR Biomed 19(4):463–475. doi:10.1002/nbm.1059
Zhang D, Hu X, Qian L, O’Callaghan JP, Hong JS (2010) Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol 41(2–3):232–241. doi:10.1007/s12035-010-8098-4
Rand SD, Prost R, Haughton V, Mark L, Strainer J, Johansen J, Kim TA, Chetty VK, Mueller W, Meyer G, Krouwer H (1997) Accuracy of single-voxel proton MR spectroscopy in distinguishing neoplastic from nonneoplastic brain lesions. AJNR Am J Neuroradiol 18(9):1695–1704
Srinivasan R, Phillips JJ, Vandenberg SR, Polley MY, Bourne G, Au A, Pirzkall A, Cha S, Chang SM, Nelson SJ (2010) Ex vivo MR spectroscopic measure differentiates tumor from treatment effects in GBM. Neuro Oncol 12(11):1152–1161. doi:10.1093/neuonc/noq075
Wehrl HF, Schwab J, Hasenbach K, Reischl G, Tabatabai G, Quintanilla-Martinez L, Jiru F, Chughtai K, Kiss A, Cay F, Bukala D, Heeren RM, Pichler BJ, Sauter AW (2013) Multimodal elucidation of choline metabolism in a murine glioma model using magnetic resonance spectroscopy and 11C-choline positron emission tomography. Cancer Res 73(5):1470–1480. doi:10.1158/0008-5472.CAN-12-2532
Stadlbauer A, Nimsky C, Gruber S, Moser E, Hammen T, Engelhorn T, Buchfelder M, Ganslandt O (2007) Changes in fiber integrity, diffusivity, and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. AJNR Am J Neuroradiol 28(3):462–469
Kamada K, Houkin K, Hida K, Matsuzawa H, Iwasaki Y, Abe H, Nakada T (1994) Localized proton spectroscopy of focal brain pathology in humans: significant effects of edema on spin-spin relaxation time. Magn Reson Med 31(5):537–540
Di Costanzo A, Scarabino T, Trojsi F, Popolizio T, Catapano D, Giannatempo GM, Bonavita S, Portaluri M, Tosetti M, d’Angelo VA, Salvolini U, Tedeschi G (2008) Proton MR spectroscopy of cerebral gliomas at 3 T: spatial heterogeneity, and tumour grade and extent. Eur Radiol 18(8):1727–1735. doi:10.1007/s00330-008-0938-5
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roder, C., Skardelly, M., Ramina, K.F. et al. Spectroscopy imaging in intraoperative MR suite: tissue characterization and optimization of tumor resection. Int J CARS 9, 551–559 (2014). https://doi.org/10.1007/s11548-013-0952-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-013-0952-1