Abstract
Introduction
A clinical decision support system (CDSS) for brain tumor classification can be used to assist in the diagnosis and grading of brain tumors. A Fast Spectroscopic Multiple Analysis (FASMA) system that uses combinations of multiparametric MRI data sets was developed as a CDSS for brain tumor classification.
Methods
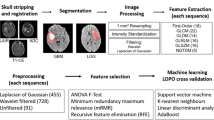
MRI metabolic ratios and spectra, from long and short TE, respectively, as well as diffusion and perfusion data were acquired from the intratumoral and peritumoral area of 126 patients with untreated intracranial tumors. These data were categorized based on the pathology, and different machine learning methods were evaluated regarding their classification performance for glioma grading and differentiation of infiltrating versus non-infiltrating lesions. Additional databases were embedded to the system, including updated literature values of the related MR parameters and typical tumor characteristics (imaging and histological), for further comparisons. Custom Graphical User Interface (GUI) layouts were developed to facilitate classification of the unknown cases based on the user’s available MR data.
Results
The highest classification performance was achieved with a support vector machine (SVM) using the combination of all MR features. FASMA correctly classified 89 and 79 % in the intratumoral and peritumoral area, respectively, for cases from an independent test set. FASMA produced the correct diagnosis, even in the misclassified cases, since discrimination between infiltrative versus non-infiltrative cases was possible.
Conclusions
FASMA is a prototype CDSS, which integrates complex quantitative MR data for brain tumor characterization. FASMA was developed as a diagnostic assistant that provides fast analysis, representation and classification for a set of MR parameters. This software may serve as a teaching tool on advanced MRI techniques, as it incorporates additional information regarding typical tumor characteristics derived from the literature.




Similar content being viewed by others
References
The brain tumor society. http://www.tbts.org
Mehndiratta A, Giesel FL (2011) Brain tumor imaging. In: Abujamra AL (ed) Diagnostic techniques and surgical management of brain tumors. InTech Publications
Pollice S, Morlino G, Capuano M, Scarabino T (2012) Brain tumors. Imaging gliomas after treatment. Spinger, Berlin, pp 3–10
Cha S (2009) Neuroimaging in neuro-oncology. Neurotherapeutics 6(3):465–477
Chiang IC, Kuo YT, Lu CY et al (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46(8):619–627
Tsolaki E, Svolos P, Kousi E, Kapsalaki E, Fountas K, Theodorou K, Tsougos I (2013) Automated differentiation of glioblastomas from intracranial metastases using 3T MR spectroscopic and perfusion data. Int J Comput Assist Radiol Surg 8(5):751- 761.
Tsougos I, Svolos P, Kousi E, Fountas K, Theodorou K, Fezoulidis I, Kapsalaki E (2012) Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging 12:423–436
Law M, Cha S, Knopp EA et al (2002) High grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222(3):715–721
Liu X, Tian W, Kolar B, Yeaney GA, Qiu X, Johnson MD et al (2011) MR diffusion tensor and perfusion-weighted imaging in preoperative grading of supratentorial nonenhancing gliomas. Neuro Oncol 13(4):447–455
Svolos P, Tsolaki E, Theodorou K, Fountas K, Kapsalaki E, Fezoulidis I, Tsougos I (2013) Classification methods for the differentiation of atypical meningiomas using diffusion and perfusion techniques at 3-T MRI. Clin Imaging 37(5):856–64
Karlsson D (2001) Aspects of the use of medical decision-support systems: the role of context in decision support. PhD thesis, Institute of Technology, Linköping University.
Tate AR, Underwood J, Acosta DM et al (2006) Development of a decision support system for diagnosis and grading of brain tumours using in vivo magnetic resonance single voxel spectra. Nucl Magn Reson Biomed 19(4):411–434
eTUMOUR Consortium, eTUMOUR: Web accessible MR decision support system for brain tumour diagnosis and prognosis. Incorporating in vivo and ex vivo genomic and metabolic data. FP6-2002-LIFESCHEALTH 503094, VI Framework Programme EC. http://www.etumour.net. Last Access 12/03/2013
Gonzalez-Velez H, Mier M, Julià-Sapé M et al (2009) HealthAgents: distributed multi-agent brain tumor diagnosis and prognosis. J Appl Intell 30:191–202
Sáez C, García-Gómez JM, Vicente J et al. (2009) Curiam BT 1.0, Decision support system for brain tumour diagnosis. In: ESMRMB congress, antalya, Turkey, EPOS Posters/Paper Posters/Info-RESO, pp 538.
De Edelenyi FS, Rubin C, Estève F, Grand S, Décorps M, Lefournier V, Le Bas JF, Rémy C (2000) A new approach for analyzing proton magnetic resonance spectroscopic images of brain tumors: nosologic images. Nat Med 6(11):1287–1289
McKnight TR, Noworolski SM, Vigneron DB, Nelson SJ (2001) An automated technique for the quantitative assessment of 3D-MRSI data from patients with glioma. J Magn Reson Imaging 13:167–177
Simonetti AW, Melssen WJ, van der Graaf M, Heerschap A, Buydens LMC (2003) A new chemometric approach for brain tumor classification using magnetic resonance imaging and spectroscopy. Anal Chem 75:5352–5361
De Vos M, Laudadio T, Simonetti AW, Heerschap A, Van Huffel S (2007) Fast nosologic imaging of the brain. J Magn Reson 184(2):292–301
Laudadio T, Martinez-Bisbal MC, Celda B, Van Huffel S (2008) Fast nosological imaging using canonical correlation analysis of brain data obtained by two-dimensional turbo spectroscopic imaging. NMR Biomed 21(4):311–321
Luts J, Laudadio T, Idema AJ et al (2009) Nosologic imaging of the brain: segmentation and classification using MRI and MRSI. NMR Biomed 22:374–390
Li Y, Sima D, Van Cauter S, Himmelreich U, Pi Y, Liu Y, van Huffel S (2013) Unsupervised nosologic imaging for glioma diagnosis. IEEE Trans Biomed Eng 60(6):1760–1763
Svolos P, Tsolaki E, Kapsalaki E, Theodorou K, Fountas K, Fezoulidis I, Tsougos I (2013) Investigating brain tumor differentiation with diffusion and perfusion metrics at 3T MRI using pattern recognition techniques. Magn Resonan Imaging 31(9): 1567–1577
Kousi E, Tsougos I, Tsolaki E, Fountas KN, Theodorou K, Fezoulidis I, Kapsalaki E, Kappas C (2012) Spectroscopic evaluation of glioma grading at 3T: the combined role of short and long TE. Sci World J 2012:546171
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
John GH, Langley P (1995) Estimating continuous distributions in Bayesian classifiers. In: Besnard P, Hanks S (eds) Proceedings of the eleventh conference on uncertainty in artificial intelligence (UAI’95). Morgan Kaufmann Publishers Inc., San Francisco, CA, USA, pp 338–345
Kazmierska J, Malicki J (2008) Application of the Naïve Bayesian classifier to optimize treatment decisions. Radiother Oncol 86(2):211–216
Cover TM, Hart PE (1967) Nearest neighbor pattern classification. Inst Electr Electron Eng Trans Inf Theory 13:21–27
Wang J, Neskovic P, Cooper LN (2007) Improving nearest neighbor rule with a simple adaptive distance measure. Pattern Recognit Lett 28(2):207–213
Fukunaga K (1990) Introduction to statistical pattern recognition, 2nd edn. Academic Press, San Diego
García-Gómez JM, Luts J, Julià-Sapé M et al (2009) Multiproject-multicenter evaluation of automatic brain tumor classification by magnetic resonance spectroscopy. Magn Reson Mater Phys 22(1):5–18
Qi H (2002) Feature selection and kNN fusion in molecular classification of multiple tumor types. In: Proceedings of the mathematics and engineering techniques in medicine and biological sciences, Las Vegas, Nevada, USA.
Li S, Harner EJ, Adjeroh DA (2011) Random KNN feature selection-a fast and stable alternative to Random Forest. BMC Bioinform 12:450
Webb AR (2002) Statistical pattern recognition. Wiley, West Sussex
Fisher RA (1936) The use of multiple measurements in taxonomic problems. Ann Eugen 7:179–188
Pöyhönen S, Arkkio A, Jover P, Hyötyniemi H (2005) Coupling pairwise support vector machines for fault classification. Control Eng Pract 13(6):759–769
Rijpkema M, Schuuring J, van der Meulen Y et al (2003) Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed 16:12–18
Majós C, Aguilera C, Cos M et al (2009) In vivo proton magnetic resonance spectroscopy of intraventricular tumours of the brain. Eur Radiol 19(8):2049–2059
Porto L, Kieslich M, Franz K et al (2011) MR spectroscopy differentiation between high and low grade astrocytomas: a comparison between paediatric and adult tumours. Eur J Paediatr Neurol 15(3):214–221
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24(10):1989–1998
Howe FA, Barton SJ, Cudlip SA et al (2003) Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 49:223–232
Castillo M, Smith JK, Kwock L (2000) Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 21(9):1645–1649
Möller-Hartmann W, Herminghaus S, Krings T et al (2002) Clinical application of proton magnetic resonance spectroscopy in the diagnosis ofintracranial mass lesions. Neuroradiology 44(5):371–381
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235(3):985–991
Price SJ (2010) Advances in imaging low-grade gliomas. Adv Tech Stand Neurosurg 35:1–34
Hakyemez B, Yildirim N, Erdoğan C et al (2006) Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation? Neuroradiology 48(10):695–702
Majós C, Alonso J, Aguilera C et al (2003) Proton magnetic resonance spectroscopy (1H MRS) of human brain tumours: assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol 13(3):582–591
Lehnhardt FG, Bock C, Röhn G, Ernestus RI, Hoehn M (2005) Metabolic differences between primary and recurrent human brain tumors: a 1H NMR spectroscopic investigation. NMR Biomed 18:371–382
Kousi E, Tsougos I, Fountas K, Theodorou K, Tsolaki E, Fezoulidis I, Kapsalaki E (2012) Distinct peak at 3.8 ppm observed by 3T MR spectroscopy in meningiomas, while nearly absent in high-grade gliomas and cerebral metastases. Mol Med Rep 5(4):1011–1018.
Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, Lim CC (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29(6):1147–1152
Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, Wan YL (2008) Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol 9:1630–1635
Zhang H, Rödiger LA, Shen T et al (2008) Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology 50(6):525–530
Server A, Josefsen R, Kulle B et al (2010) Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol 51(3):316–325
Li G, Yang J, Ye C, Geng D (2006) Degree prediction of malignancy in brain glioma using support vector machines. Comput Biol Med 36(3):313–325
Zacharaki EI, Kanas VG, Davatzikos C (2011) Investigating machine learning techniques for MRI-based classification of brain neoplasms. Int J Comput Assist Radiol Surg 6(6):821–828
Devos A et al (2005) The use of multivariate MR imaging intensities versus metabolic data from MR spectroscopic imaging for brain tumour classification. J Magn Reson 173(2):218–228
Di Costanzo A, Scarabino T, Trojsi F et al (2008) Proton MR spectroscopy of cerebral gliomas at 3 T: spatial heterogeneity, and tumour grade and extent. Eur Radiol 18:1727–1735
Zonari P, Baraldi P, Crisi G (2007) Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology 49(10):795–803
Luts J, Heerschap A, Suykens JAK, Van Huffel S (2007) A combined MRI and MRSI based multiclass system for brain tumour recognition using LS-SVMs with class probabilities and feature selection. Artif Intell Med 40(2):87–102
Ben-Hur A, Weston J (2010) A user’s guide to support vector machines. Methods Mol Biol 609:223–239
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27(3):475–487
Ginat DT, Mangla R et al (2010) Correlation of diffusion and perfusion MRI with Ki-67 in high-grade meningiomas. AJR Am J Roentgenol 195(6):1391–1395
Fan G, Sun B, Wu Z, Guo Q, Guo Y (2004) In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin Radiol 59(1):77–85
Chernov MF, Kubo O, Hayashi M et al (2005) Proton MRS of the peritumoral brain. J Neurol Sci 228(2):137–142
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Tsolaki, E., Svolos, P., Kousi, E. et al. Fast spectroscopic multiple analysis (FASMA) for brain tumor classification: a clinical decision support system utilizing multi-parametric 3T MR data. Int J CARS 10, 1149–1166 (2015). https://doi.org/10.1007/s11548-014-1088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-014-1088-7