Abstract
Purpose
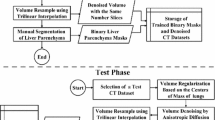
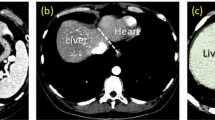
Automated liver segmentation from a postmortem computed tomography (PMCT) volume is a challenging problem owing to the large deformation and intensity changes caused by severe pathology and/or postmortem changes. This paper addresses this problem by a novel segmentation algorithm using a statistical shape model (SSM) for a postmortem liver.
Methods
The location and shape parameters of a liver are directly estimated from a given volume by the proposed SSM-guided expectation–maximization (EM) algorithm without any spatial standardization that might fail owing to the large deformation and intensity changes. The estimated location and shape parameters are then used as a constraint of the subsequent fine segmentation process based on graph cuts. Algorithms with eight different SSMs were trained using 144 in vivo and 32 postmortem livers, and the segmentation algorithm was tested on 32 postmortem livers in a twofold cross validation manner. The segmentation performance is measured by the Jaccard index (JI) between the segmentation result and the true liver label.
Results
The average JI of the segmentation result with the best SSM was 0.8501, which was better compared with the results obtained using conventional SSMs and the results of the previous postmortem liver segmentation with statistically significant difference.
Conclusions
We proposed an algorithm for automated liver segmentation from a PMCT volume, in which an SSM-guided EM algorithm estimated the location and shape parameters of a liver in a given volume accurately. We demonstrated the effectiveness of the proposed algorithm using actual postmortem CT volumes.





















Similar content being viewed by others
References
Ezawa H, Yoneyama R, Kandatsu S, Yoshikawa K, Tsujii H, Harigaya K (2003) Introduction of autopsy imaging redefines the concept of autopsy: 37 cases of clinical experience. Pathol Int 53(12):865–873. doi:10.1046/j.1440-1827.2003.01573.x
Okuda T, Shiotani S, Sakamoto N, Kobayashi T (2013) Background and current status of postmortem imaging in Japan: short history of “autopsy imaging (Ai)”. Forensic Sci Int 225(1):3–8. doi:10.1016/j.forsciint.2012.03.010
Saito A, Shimizu A, Watanabe H, Yamamoto S, Kobatake H (2013) Automated liver segmentation from a CT volume of a cadaver using a statistical shape model. Int J Comput Assist Radiol Surg 8(Suppl 1):S48–S49. doi:10.1007/s11548-013-0850-6
Punia R, Singh S (2013) Review on machine learning techniques for automatic segmentation of liver images. Int J Adv Res Comput Sci Softw Eng 3(4):666–670
Park H, Bland PH, Meyer CR (2003) Construction of an abdominal probabilistic atlas and its application in segmentation. IEEE Trans Med Imaging 22(4):483–492. doi:10.1109/TMI.2003.809139
Shimizu A, Ohno R, Ikegami T, Kobatake H, Nawano S, Smutek D (2007) Segmentation of multiple organs in non-contrast 3D abdominal CT images. Int J Comput Assist Radiol Surg 2(3–4):135–142. doi:10.1007/s11548-007-0135-z
Chu C, Oda M, Kitasaka T, Misawa K, Fujiwara M, Hayashi Y, Nimura Y, Rueckert D, Mori K (2013) Multi-organ segmentation based on spatially-divided probabilistic atlas from 3D abdominal CT images. In: Medical image computing and computer-assisted intervention. Springer, pp 165–172. doi:10.1007/978-3-642-40763-5_21
Wolz R, Chu C, Misawa K, Fujiwara M, Mori K, Rueckert D (2013) Automated abdom inal multi-organ segmentation with subject-specific atlas generation. IEEE Trans Med Imaging 32(9):1723–1730. doi:10.1109/TMI.2013.2265805
Umetsu S, Shimizu A, Watanabe H, Kobatake H, Nawano S (2014) An automated segmentation algorithm for CT volumes of livers with atypical shapes and large pathological lesions. IEICE Trans Inf Syst 97(4):951–963. doi:10.1587/transinf.E97.D.951
Tong T, Wolz R, Wang Z, Gao Q, Misawa K, Fujiwara M, Mori K, Hajnal JV, Rueckert D (2015) Discriminative dictionary learning for abdominal multi-organ segmentation. Med Image Anal 23(1):92–104. doi:10.1016/j.media.2015.04.015
Kainmüller D, Lange T, Lamecker H (2007) Shape constrained automatic segmentation of the liver based on a heuristic intensity model. In: Proceedings of MICCAI Workshop 3D segmentation in the clinic: a grand challenge, pp 109–116
Heimann T, van Ginneken B, Styner MA, Arzhaeva Y, Aurich V, Bauer C, Beck A, Becker C, Beichel R, Bekes G, Bello F, Binnig G, Bischof H, Bornik A, Cashman PMM, Chi Y, Cordova A, Dawant BM, Fidrich M, Furst JD, Furukawa D, Grenacher L, Hornegger J, Kainmller D, Kitney RI, Kobatake H, Lamecker H, Lange T, Lee J, Lennon B, Li R, Li S, Meinzer HP, Nemeth G, Raicu DS, Rau AM, van Rikxoort EM, Rousson M, Rusko L, Saddi KA, Schmidt G, Seghers D, Shimizu A, Slagmolen P, Sorantin E, Soza G, Susomboon R, Waite JM, Wimmer A, Wolf I (2009) Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Trans Med Imaging 28(8):1251–1265. doi:10.1109/TMI.2009.2013851
Heimann T, Meinzer HP (2009) Statistical shape models for 3D medical image segmentation: a review. Med Image Anal 13(4):543–563. doi:10.1016/j.media.2009.05.004
Cootes TF, Taylor CJ, Cooper DH, Graham J (1995) Active shape models-their training and application. Comput Vis Image Underst 61(1):38–59. doi:10.1006/cviu.1995.1004
Cremers D, Rousson M, Deriche R (2007) A review of statistical approaches to level set segmentation: integrating color, texture, motion and shape. Int J Comput Vis 72(2):195–215. doi:10.1007/s11263-006-8711-1
Boykov Y, Veksler O, Zabih R (2001) Fast approximate energy minimization via graph cuts. IEEE Trans Pattern Anal Mach Intell 23(11):1222–1239. doi:10.1109/34.969114
Tomoshige S, Oost E, Shimizu A, Watanabe H, Nawano S (2014) A conditional statistical shape model with integrated error estimation of the conditions; application to liver segmentation in non-contrast CT images. Med Image Anal 18(1):130–143. doi:10.1016/j.media.2013.10.003
Okada T, Linguraru MG, Hori M, Summers RM, Tomiyama N, Sato Y (2015) Abdominal multi-organ segmentation from CT images using conditional shape-location and unsupervised intensity priors. Med Image Anal 26(1):1–18. doi:10.1016/j.media.2015.06.009
Linguraru MG, Pura JA, Pamulapati V, Summers RM (2012) Statistical 4D graphs for multi-organ abdominal segmentation from multiphase CT. Med Image Anal 16(4):904–914. doi:10.1016/j.media.2012.02.001
Saito A, Shimizu A, Watanabe H, Yamamoto S, Nawano S, Kobatake H (2014) Statistical shape model of a liver for autopsy imaging. Int J Comput Assist Radiol Surg 9(2):269–281. doi:10.1007/s11548-013-0923-6
Cremers D (2006) Dynamical statistical shape priors for level set-based tracking. IEEE Trans Pattern Anal Mach Intell 28(8):1262–1273. doi:10.1109/TPAMI.2006.161
Uchida Y, Shimizu A, Kobatake H, Nawano S, Shinozaki K (2010) A comparative study of statistical shape models of the pancreas. Int J Comput Assist Radiol Surg 5(Suppl 1):S385–S387. doi:10.1007/s11548-010-0469-9
Pohl KM, Fisher J, Bouix S, Shenton M, McCarley RW, Grimson WEL, Kikinis R, Wells WM (2007) Using the logarithm of odds to define a vector space on probabilistic atlases. Med Image Anal 11(5):465–477. doi:10.1016/j.media.2007.06.003
Sanjay-Gopal S, Hebert T (1998) Bayesian pixel classification using spatially variant finite mixtures and the generalized EM algorithm. IEEE Trans Image Process 7(7):1014–1028. doi:10.1109/83.701161
Dempster AP, Laird NM, Rubin DB (1977) Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Ser B (methodol). doi:10.2307/2984875
Shimizu A, Nakagomi K, Narihira T, Kobatake H, Nawano S, Shinozaki K, Ishizu K, Togashi K (2010) Automated segmentation of 3D CT images based on statistical atlas and graph cuts. In: Medical computer vision. Recognition techniques and applications in medical imaging. Springer, pp 214–223. doi:10.1007/978-3-642-18421-5_21
Saito T, Toriwaki JI (1994) New algorithms for euclidean distance transformation of an n-dimensional digitized picture with applications. Pattern Recognit 27(11):1551–1565. doi:10.1016/0031-3203(94)90133-3
Maurer CR Jr, Qi R, Raghavan V (2003) A linear time algorithm for computing exact euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans Pattern Anal Mach Intell 25(2):265–270. doi:10.1109/TPAMI.2003.1177156
Demšar J (2006) Statistical comparisons of classifiers over multiple data. J Mach Learn Res 7:1–30
Hasegawa I, Shimizu A, Saito A, Püschel K, Suzuki H, Vogel H, Heinemann A (2016) Evaluation of post-mortem lateral cerebral ventricle changes using sequential scans dur-ing post-mortem computed tomography. Int J Legal Med. doi:10.1007/s00414-016-1327-2
Saito A, Nawano S, Shimizu A (2016) Joint optimization of segmentation and shape prior from level-set-based statistical shape model, and its application to the automated segmentation of abdominal organs. Med Image Anal 28:46–65. doi:10.1016/j.media.2015.11.003
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Number 15J08775 and MEXT KAKENHI Grant Number 26108002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration, as revised in 2008(5).
Informed consent
For this type of study, formal consent is not required.
Appendix: Calculation of the gradient \(\nabla f({\varvec{t}}')\) and \(\nabla g({\varvec{\alpha }}')\)
Appendix: Calculation of the gradient \(\nabla f({\varvec{t}}')\) and \(\nabla g({\varvec{\alpha }}')\)
Because of the difficulties faced in the analytical computation of the derivative of \(\phi \) with respect to \({\varvec{t}}\), the gradient \(\nabla f({\varvec{t}}')\) is obtained by difference approximation.
where \(\varDelta _x = [\delta ,0,0]^\top \), \(\varDelta _y = [0,\delta ,0]^\top \), and \(\varDelta _z = [0,0,\delta ]^\top \) with \(\delta = 1\) voxel. The partial derivative of \(g({\varvec{\alpha }}) = Q\left( {\varTheta ',{\varvec{\alpha }},{\varvec{t}}'}\big |{\varTheta ',{\varvec{\alpha }}',{\varvec{t}}'}\right) \) in terms of \(\alpha _j\) used in Eq. (25) is derived as follows:



In Eq. (39), we used the fact that, for an arbitrary function h(v), the following equation holds for the derivative of the logarithm of the sigmoid function \(\ln {\varsigma _a(h(v))}\):
and for the derivative of \(\ln \{1-\varsigma _a(h(v))\}\):
Rights and permissions
About this article
Cite this article
Saito, A., Yamamoto, S., Nawano, S. et al. Automated liver segmentation from a postmortem CT scan based on a statistical shape model. Int J CARS 12, 205–221 (2017). https://doi.org/10.1007/s11548-016-1481-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-016-1481-5