Abstract
Purpose
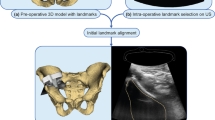
Ultrasound (US) is a safer alternative to X-rays for bone imaging, and its popularity for orthopedic surgical navigation is growing. Routine use of intraoperative US for navigation requires fast, accurate and automatic alignment of tracked US to preoperative computed tomography (CT) patient models. Our group previously investigated image segmentation and registration to align untracked US to CT of only the partial pelvic anatomy. In this paper, we extend this to study the performance of these previously published techniques over the full pelvis in a tracked framework, to characterize their suitability in more realistic scenarios, along with an additional simplified segmentation method and similarity metric for registration.
Method
We evaluated phase symmetry segmentation, and Gaussian mixture model (GMM) and coherent point drift (CPD) registration methods on a pelvic phantom augmented with human soft tissue images. Additionally, we proposed and evaluated a simplified 3D bone segmentation algorithm we call Shadow–Peak (SP), which uses acoustic shadowing and peak intensities to detect bone surfaces. We paired this with a registration pipeline that optimizes the normalized cross-correlation (NCC) between distance maps of the segmented US–CT images.
Results
SP segmentation combined with the proposed NCC registration successfully aligned tracked US volumes to the preoperative CT model in all trials, in contrast to the other techniques. SP with NCC achieved a median target registration error (TRE) of 2.44 mm (maximum 4.06 mm), when imaging all three anterior pelvic structures, and a mean runtime of 27.3 s. SP segmentation with CPD registration was the next most accurate combination: median TRE of 3.19 mm (maximum 6.07 mm), though a much faster runtime of 4.2 s.
Conclusion
We demonstrate an accurate, automatic image processing pipeline for intraoperative alignment of US–CT over the full pelvis and compare its performance with the state-of-the-art methods. The proposed methods are amenable to clinical implementation due to their high accuracy on realistic data and acceptably low runtimes.







Similar content being viewed by others
References
Ecker T, Jost J, Cullmann J, Zech W, Djonov V, Keel M, Benneker L, Bastian J (2017) Percutaneous screw fixation of the iliosacral joint: a case-based preoperative planning approach reduces operating time and radiation exposure. Injury 48(8):1825–1830
Schueler BA (2010) Operator shielding: how and why. Tech Vasc Interv Radiol 13(3):167–171
Daanen V, Tonetti J, Troccaz J (2004) A fully automated method for the delineation of osseous interface in ultrasound images. In: Medical image computing and computer-assisted intervention—MICCAI 2004, 3216, pp 549–557
Tonetti J, Carrat L, Blendea S, Merloz P, Troccaz J, Lavalle S, Chirossel JP (2001) Clinical results of percutaneous pelvic surgery: computer assisted surgery using ultrasound compared to standard fluoroscopy. Comput Aided Surg 6(4):204–211
Brendel B, Rick SWA, Stockheim M, Ermert H (2002) Registration of 3D CT and ultrasound datasets of the spine using bone structures. Comput Aided Surg 7(3):146–155
Penney G, Barratt D, Chan C, Slomczykowski M, Carter T, Edwards P, Hawkes D (2006) Cadaver validation of intensity-based ultrasound to CT registration. Med Image Anal 10(3):385–395
Barratt D, Penney G, Chan C, Slomczykowski M, Carter T, Edwards P, Hawkes D (2006) Self-calibrating 3D-ultrasound-based bone registration for minimally invasive orthopedic surgery. IEEE Trans Med Imaging 25(3):312–323
Ghanavati S, Mousavi P, Fichtinger G, Foroughi P, Abolmaesumi P (2010) Multi-slice to volume registration of ultrasound data to a statistical atlas of human pelvis. In: Proceedings of SPIE, vol 7625, p 7625–7625–10
Ghanavati S, Mousavi P, Fichtinger G, Abolmaesumi P (2011) Phantom validation for ultrasound to statistical shape model registration of human pelvis. In: Proceedings of SPIE, vol 7964, pp 7964–7964–8
Hacihaliloglu I, Abugharbieh R, Hodgson AJ, Rohling RN (2009) Bone surface localization in ultrasound using image phase-based features. Ultrasound Med Biol 35(9):1475–1487
Hacihaliloglu I, Abugharbieh R, Hodgson AJ, Rohling RN, Guy P (2012) Automatic bone localization and fracture detection from volumetric ultrasound images using 3-D local phase features. Ultrasound Med Biol 38(1):128–144
Hacihaliloglu I, Abugharbieh R, Hodgson AJ, Rohling RN (2011) Automatic adaptive parameterization in local phase feature-based bone segmentation in ultrasound. Ultrasound Med Biol 37(10):1689–1703
Hacihaliloglu I, Wilson DR, Gilbart M, Hunt MA, Abolmaesumi P (2013) Non-iterative partial view 3D ultrasound to CT registration in ultrasound-guided computer-assisted orthopedic surgery. Int J Comput Assist Radiol Surg 8(2):157–168
Brounstein A, Hacihaliloglu I, Guy P, Hodgson A, Abugharbieh R (2011) Towards real-time 3D US to CT bone image registration using phase and curvature feature based GMM matching. In: Medical image computing and computer intervention—MICCAI 2011, vol 14, pp 235–242
Hacihaliloglu I, Brounstein A, Guy P, Hodgson A, Abugharbieh R (2012) 3D ultrasound-CT registration in orthopaedic trauma using GMM registration with optimized particle simulation-based data reduction. In: Medical image computing and computer-assisted intervention—MICCAI 2012, vol 15, pp 82–89
Brounstein A, Hacihaliloglu I, Guy P, Hodgson A, Abugharbieh R (2015) Fast and accurate data extraction for near real-time registration of 3-D ultrasound and computed tomography in orthopedic surgery. Ultrasound Med Biol 41(12):3194–3204
Wein W, Brunke S, Khamene A, Callstrom MR, Navab N (2008) Automatic CT-ultrasound registration for diagnostic imaging and image-guided intervention. Med Image Anal 12(5):577–585
Wein W, Karamalis A, Baumgartner A, Navab N (2015) Automatic bone detection and soft tissue aware ultrasound–CT registration for computer-aided orthopedic surgery. Int J Comput Assist Radiol Surg 10(6):971–979
Salehi M, Prevost R, Moctezuma JL, Navab N, Wein W (2017) Precise ultrasound bone registration with learning-based segmentation and speed of sound calibration. In: Descoteaux M, Maier-Hein L, Franz A, Jannin P, Collins DL, Duchesne S (eds) Medical Image Computing and Computer-assisted intervention—MICCAI 2017. Springer, Cham, pp 682–690
Brattain LJ, Telfer BA, Dhyani M, Grajo JR, Samir AE (2018) Machine learning for medical ultrasound: status, methods, and future opportunities. Abdom Radiol 43(4):786–799
Hacihaliloglu I, Abugharbieh R, Hodgson A, Rohling R (2008) Bone segmentation and fracture detection in ultrasound using 3d local phase features. In: Medical image computing and computer-assisted intervention—MICCAI 2008. Springer, Berlin, pp 287–295
Myronenko A, Song X (2010) Point set registration: coherent point drift. IEEE Trans Pattern Anal Mach Intell 32(12):2262–2275
Pandey P, Abugharbieh R, Hodgson AJ (2017) Trackerless 3d ultrasound stitching for computer-assisted orthopaedic surgery and pelvic fractures. In: CAOS 2017, EasyChair, EPiC Series in Health Sciences, vol 1, pp 318–321
Quader N, Hodgson A, Abugharbieh R (2014) Confidence weighted local phase features for robust bone surface segmentation in ultrasound. In: MICCAI workshop on clinical image-based procedures, pp 76–83
Karamalis A, Wein W, Klein T, Navab N (2012) Ultrasound confidence maps using random walks. Med Image Anal 16(6):1101–1112
Hacihaliloglu I, Guy P, Hodgson AJ, Abugharbieh R (2015) Automatic extraction of bone surfaces from 3D ultrasound images in orthopaedic trauma cases. Int J Comput Assist Radiol Surg 10(8):1279–1287
Maurer C, Qi Rensheng, Raghavan V (2003) A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans Pattern Anal Mach Intell 25(2):265–270
Madsen EL, Hobson MA, Shi H, Varghese T, Frank GR (2005) Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys Med Biol 50(23):5597–5618
Lasso A, Heffter T, Rankin A, Pinter C, Ungi T, Fichtinger G (2014) PLUS: open-source toolkit for ultrasound-guided intervention systems. IEEE Trans Biomed Eng 61(10):2527–37
Kikinis R, Pieper SD, Vosburgh KG (2014) 3D slicer: a platform for subject-specific image analysis, visualization, and clinical support. Springer, New York, pp 277–289
Ungi T, Lasso A, Fichtinger G (2016) Open-source platforms for navigated image-guided interventions. Med Image Anal 33:181–186
Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW (2010) Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29(1):196–205
Baka N, Leenstra S, van Walsum T (2017) Ultrasound aided vertebral level localization for lumbar surgery. IEEE Trans Med Imaging 36(10):1–1
Bates P, Starr A, Reinert C (2010) The percutaneous treatment of pelvic and acetabular fractures. Independent Publisher
Shams R, Sadeghi P, Kennedy R, Hartley R (2010) A survey of medical image registration on multicore and the GPU. IEEE Signal Process Mag 27(2):50–60
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the Natural Sciences and Engineering Research Council (Grant Number: CHRP 478466-15) and the Canadian Institutes of Health Research (Grant Number: CPG-140180).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This article does not contain patient data.
Rights and permissions
About this article
Cite this article
Pandey, P., Guy, P., Hodgson, A.J. et al. Fast and automatic bone segmentation and registration of 3D ultrasound to CT for the full pelvic anatomy: a comparative study. Int J CARS 13, 1515–1524 (2018). https://doi.org/10.1007/s11548-018-1788-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-018-1788-5