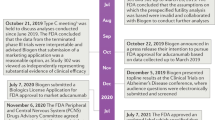
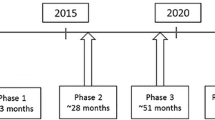
Abstract
Alzheimer’s disease (AD), the devastating and most prevailing underlying cause for age-associated dementia, has no effective disease-modifying treatment. The last approved drug for the relief of AD symptoms was in 2003. The recent approval of sodium oligomannate (GV-971, 2019) in China and the human antibody aducanumab in the USA (ADUHELM, 2021) therefore represent significant breakthroughs, albeit ones that are fraught with controversy. Here, we explore potential scientific ethics issues associated with GV-971 and aducanumab’s development and approval. While these issues may be belied by socioeconomic and political complexities in the heady business of commercial drug development, they are of fundamental importance to scientific integrity and ultimately, welfare of patients. We posit that the push for approval of both AD drugs based on incomplete research and unconvincing marginal effectiveness is ethically unsound. Regardless of how both these drugs shall perform in the market for the years to come, the scientific ethics issues and potentially questionable research practices should therefore be duly noted and lessons learned.
Similar content being viewed by others
Notes
Searches with the term “oligomannate” or “GV-971” conducted at ClinicalTrials.gov (https://clinicaltrials.gov) returned nine projects in various stages of recruitment/completion. Two of these are Phase I trials with healthy participants (NCT02986529 and NCT03715114 (see Table 1) to examine bioavailability and pharmacodynamics. Six of these are Phase I to IV trials on AD and one on post-stroke cognitive impairment. Searches with the term “oligomannate” or “GV-971” made at the Chinese Clinical Trial Registry (CHICTR) (www.chictr.org.cn) returned four projects: ChiCTR2100042680 and ChiCTR2100053873 are on Parkinson’s disease, both 2021), while ChiCTR2000039219 (A multicentre observational study of the efficacy and safety of Sodium Oligomannate Capsules in the treatment of Alzheimer's disease, 2020) and ChiCTR2100047830 (Effects of GV-971 and Donepezil in Alzheimer's Disease, 2021) are on AD.
This requirement is according to that of the US FDA, and may not be one adopted by China’s National Medical Products Administration (NMPA). The “conditional approval” for GV-971 in AD treatment by NMPA would be in accordance with the following that is paraphrased from a slide presented by Xiaoping Cao, Pfizer Senior Director and Head of Global CMC for China at the PharmaLink 2021 conference, and shown in a blogpost by Jerry Chapman (https://redica.com/pharma-an-inside-look-at-chinas-regulatory-and-drug-approval-processes). “During local clinical trial, the clinical data can predict its efficacy and clinical benefits for target indications that are serious and life-threatening with unmet clinical needs, urgent need for drugs to treat rare diseases, drugs urgently needed in public health and vaccines with special approval”. The first two of these would be descriptive of AD.
Geng (and her work on GV-971) is among several other prominent Chinese researchers investigated recently by a committee comprising representatives from several Chinese ministries. According to a “Notice on the Investigation and Handling of Suspected Fraudulent Papers” issued by the Ministry of Science and Technology of China on Jan 2021, no fraud on Geng’s part was found. However, there were instances of misuse of pictures in her papers and remedial education and scientific research integrity reminders shall be implemented.
The dosing used in these trials are complicated by protocol amendments, particularly that to a subset of the test participants who are carriers of the apolipoprotein E (APOE) ε4 allele who are more susceptible to ARIA. Initially by APOE ε4 carriers were only to be tested on the lower doses up to 6 mg/kg, but as of Mar 2017 these were also to be titrated to 10 mg/kg.
PET-SUVR refers to the imaging technique for quantitative evaluation of amyloid load with positron emission tomography (PET) with standardized uptake value ratio (SUVR).
Biogen’s powerpoint presentation file (2019). EMERGE and ENGAGE Topline Results: Two Phase III Studies to Evaluate Aducanumab in Patients With Early Alzheimer’s Disease. (https://investors.biogen.com/static-files/ddd45672-9c7e-4c99-8a06-3b557697c06f).
Initially indicated for all stages of AD, but later revised to include only patients with MCI and mild AD, which is in line with the cohort profile investigated in the phase III trials.
The more technically inclined reader is referred to these papers for further detail analyses.
The Centers for Medicare & Medicaid Services (CMS) has announced a preliminary National Coverage Determination (NCD) restricting Medicare coverage of the aducanumab to patients who are participating in approved placebo-controlled clinical trials.
References
Alexander, G. C., Emerson, S., & Kesselheim, A. S. (2021a). Evaluation of Aducanumab for Alzheimer disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA, 325(17), 1717–1718. https://doi.org/10.1001/jama.2021.3854
Alexander, G. C., Knopman, D. S., Emerson, S. S., Ovbiagele, B., Kryscio, R. J., Perlmutter, J. S., & Kesselheim, A. S. (2021b). Revisiting FDA approval of Aducanumab. The New England Journal of Medicine, 385(9), 769–771. https://doi.org/10.1056/NEJMp2110468
Alzforum. (2021). Solanezumab. Retrieved May 2022, from https://www.alzforum.org/therapeutics/solanezumab
Association, A. (2021). 2021 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia, 17(3), 327–406. https://doi.org/10.1002/alz.12328
Bik, E. (2021). Cassava Sciences: Of stocks and blots. Retrieved May 2022, from https://scienceintegritydigest.com/2021/08/27/cassava-sciences-of-stocks-and-blots/
Brookmeyer, R., Corrada, M. M., Curriero, F. C., & Kawas, C. (2002). Survival following a diagnosis of Alzheimer disease. Archives of Neurology, 59(11), 1764–1767. https://doi.org/10.1001/archneur.59.11.1764
Budd Haeberlein, S., Aisen, P. S., Barkhof, F., Chalkias, S., Chen, T., Cohen, S., Dent, G., Hansson, O., Harrison, K., von Hehn, C., Iwatsubo, T., Mallinckrodt, C., Mummery, C. J., Muralidharan, K. K., Nestorov, I., Nisenbaum, L., Rajagovindan, R., Skordos, L., Tian, Y., … Sandrock, A. (2022). Two randomized phase 3 studies of Aducanumab in early Alzheimer’s disease. Journal of Prevention of Alzheimer’s Disease, 9(2), 197–210. https://doi.org/10.14283/jpad.2022.30
Budd Haeberlein, S., O’Gorman, J., Chiao, P., Bussière, T., von Rosenstiel, P., Tian, Y., Zhu, Y., von Hehn, C., Gheuens, S., Skordos, L., Chen, T., & Sandrock, A. (2017). Clinical development of Aducanumab, an anti-Aβ human monoclonal antibody being investigated for the treatment of early Alzheimer’s disease. The Journal of Prevention of Alzheimer’s Disease, 4(4), 255–263. https://doi.org/10.14283/jpad.2017.39
Cassava Sciences. (2021). Cassava sciences’ Simufilam improves cognition and behavior in Alzheimer’s disease in interim analysis of open-label study. Retrieved May 2022, from https://www.cassavasciences.com/news-releases/news-release-details/cassava-sciences-simufilam-improves-cognition-and-behavior?source=content_type%3Areact%7Cfirst_level_url%3Aarticle%7Csection%3Amain_content%7Cbutton%3Abody_link
Chertkow, H., Rockwood, K., Hogan, D. B., Phillips, N., Montero-Odasso, M., Amanullah, S., Black, S., Bocti, C., Borrie, M., Feldman, H., Freedman, M., Hsiung, R., Kirk, A., Masellis, M., Nygaard, H., Rajji, T., & Verret, L. (2021). Consensus statement regarding the application of Biogen to health Canada for approval of Aducanumab. Canadian Geriatrics Journal, 24(4), 373–378. https://doi.org/10.5770/cgj.24.570
Chiong, W., Tolchin, B. D., Bonnie, R. J., Busl, K., Cruz-Flores, S., Epstein, L. G., Greene, E. P., Illes, J., Kirschen, M., Larriviere, D. G., Mantri, S., Rubin, M. A., Stern, B. J., Taylor, L. P. & Ethics Law and Humanities Committee. (2021). Decisions with patients and families regarding aducanumab in Alzheimer disease, with recommendations for consent: AAN position statement. Neurology. https://doi.org/10.1212/WNL.0000000000013053
Cummings, J., Aisen, P., Lemere, C., Atri, A., Sabbagh, M., & Salloway, S. (2021). Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Research & Therapy, 13(1), 98. https://doi.org/10.1186/s13195-021-00838-z
Cummings, J. L., Morstorf, T., & Zhong, K. (2014). Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer's Research & Therapy, 6(4), 37. https://doi.org/10.1186/alzrt269
Doggrell, S. A. (2018). Grasping at straws: The failure of solanezumab to modify mild Alzheimer’s disease. Expert Opinion on Biological Therapy, 18(12), 1189–1192. https://doi.org/10.1080/14712598.2018.1543397
Dunn, B., Stein, P., & Cavazzoni, P. (2021). Approval of Aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Internal Medicine, 181(10), 1276–1278. https://doi.org/10.1001/jamainternmed.2021.4607
FDA. (2021). FDA grants accelerated approval for Alzheimer’s drug. Retrieved May 2022, from https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
US FDA. Aducanumab for the treatment of Alzheimer’s disease: Presentation. US Food and Drug Administration.
Feustel, A. C., MacPherson, A., Fergusson, D. A., Kieburtz, K., & Kimmelman, J. (2020). Risks and benefits of unapproved disease-modifying treatments for neurodegenerative disease. Neurology, 94(1), e1–e14. https://doi.org/10.1212/WNL.0000000000008699
Fleck, L. M. (2021). Alzheimer’s and Aducanumab: Unjust profits and false hopes. Hastings Center Report, 51(4), 9–11. https://doi.org/10.1002/hast.1264
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer’s disease: The amyloid cascade hypothesis. Science, 256(5054), 184–185. https://doi.org/10.1126/science.1566067
Herman, B. (2021). Biogen pulled Aduhelm paper after JAMA demanded edits. Retrieved May 2022, from https://www.axios.com/biogen-jama-aduhelm-clinical-trial-results-publish-fc7c2876-a684-4bfc-8462-4165f57d735a.html
Howard, R., & Liu, K. Y. (2020). Questions EMERGE as Biogen claims Aducanumab turnaround. Nature Reviews Neurology, 16(2), 63–64. https://doi.org/10.1038/s41582-019-0295-9
Joseph, A. (2019). New Alzheimer’s therapy approved in China, delivering a surprise but raising questions. Retrieved May 2022, from https://www.statnews.com/2019/11/04/a-new-alzheimers-therapy-is-approved-in-china-delivering-a-surprise-for-the-field-but-also-questions/
Karlawish, J., & Grill, J. D. (2021). The approval of Aduhelm risks eroding public trust in Alzheimer research and the FDA. Nature Reviews Neurology, 17(9), 523–524. https://doi.org/10.1038/s41582-021-00540-6
Kastanenka, K. V., Bussiere, T., Shakerdge, N., Qian, F., Weinreb, P. H., Rhodes, K., & Bacskai, B. J. (2016). Immunotherapy with Aducanumab restores calcium homeostasis in Tg2576 mice. Journal of Neuroscience, 36(50), 12549–12558. https://doi.org/10.1523/JNEUROSCI.2080-16.2016
Knopman, D. S., Jones, D. T., & Greicius, M. D. (2021). Failure to demonstrate efficacy of Aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s and Dementia, 17(4), 696–701. https://doi.org/10.1002/alz.12213
Lee, K., Bacchetti, P., & Sim, I. (2008). Publication of clinical trials supporting successful new drug applications: A literature analysis. PLoS Medicine, 5(9), e191. https://doi.org/10.1371/journal.pmed.0050191
Liu, K. Y., Schneider, L. S., & Howard, R. (2021). The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry, 8(11), 1013–1016. https://doi.org/10.1016/S2215-0366(21)00197-8
Lozupone, M., Solfrizzi, V., D’Urso, F., Di Gioia, I., Sardone, R., Dibello, V., Stallone, R., Liguori, A., Ciritella, C., Daniele, A., Bellomo, A., Seripa, D., & Panza, F. (2020). Anti-amyloid-β protein agents for the treatment of Alzheimer’s disease: An update on emerging drugs. Expert Opinion on Emerging Drugs, 25(3), 319–335. https://doi.org/10.1080/14728214.2020.1808621
Lu, J., Pan, Q., Zhou, J., Weng, Y., Chen, K., Shi, L., Zhu, G., Chen, C., Li, L., Geng, M., & Zhang, Z. (2022). Pharmacokinetics, distribution, and excretion of sodium oligomannate, a recently approved anti-Alzheimer’s disease drug in China. Journal of Pharmaceutical Analysis, 12(1), 145–155. https://doi.org/10.1016/j.jpha.2021.06.001
Mahase, E. (2021). Aducanumab: European agency rejects Alzheimer’s drug over efficacy and safety concerns. BMJ, 375, n3127. https://doi.org/10.1136/bmj.n3127
Megur, A., Baltriukienė, D., Bukelskienė, V., & Burokas, A. (2020). The microbiota-gut-brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients. https://doi.org/10.3390/nu13010037
Mullard, A. (2021). Landmark Alzheimer’s drug approval confounds research community. Nature, 594(7863), 309–310. https://doi.org/10.1038/d41586-021-01546-2
Musiek, E. S., Gomez-Isla, T., & Holtzman, D. M. (2021). Aducanumab for Alzheimer disease: The amyloid hypothesis moves from bench to bedside. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI154889
Panza, F., Lozupone, M., Logroscino, G., & Imbimbo, B. P. (2019). A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nature Reviews Neurology, 15(2), 73–88. https://doi.org/10.1038/s41582-018-0116-6
Rao, Y. (2020). Omission of previous publications by an author should be corrected. Cell Research, 30(9), 819. https://doi.org/10.1038/s41422-020-0344-3
Resnik, D. B., & Shamoo, A. E. (2011). The Singapore statement on research integrity. Accountability in Research, 18(2), 71–75. https://doi.org/10.1080/08989621.2011.557296
Rising, K., Bacchetti, P., & Bero, L. (2008). Reporting bias in drug trials submitted to the Food and Drug Administration: Review of publication and presentation. PLoS Medicine, 5(11), e217. https://doi.org/10.1371/journal.pmed.0050217. discussion e217.
Rogers, S. L., Doody, R. S., Mohs, R. C., Friedhoff, L. T. & Donepezil Study Group. (1998). Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Archives of Internal Medicine, 158(9), 1021–1031. https://doi.org/10.1001/archinte.158.9.1021
Rutsch, A., Kantsjö, J. B., & Ronchi, F. (2020). The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2020.604179
Sachs, R. E., & Bagley, N. (2021). Medicare coverage of Aducanumab: Implications for state budgets. The New England Journal of Medicine, 385(22), 2019–2021. https://doi.org/10.1056/NEJMp2115297
Salloway, S., Chalkias, S., Barkhof, F., Burkett, P., Barakos, J., Purcell, D., Suhy, J., Forrestal, F., Tian, Y., Umans, K., Wang, G., Singhal, P., Budd Haeberlein, S., & Smirnakis, K. (2022). Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating Aducanumab in patients with early Alzheimer disease. JAMA Neurology, 79(1), 13–21. https://doi.org/10.1001/jamaneurol.2021.4161
Salloway, S., & Cummings, J. (2021). Aducanumab, amyloid lowering, and slowing of Alzheimer disease. Neurology, 97(11), 543–544. https://doi.org/10.1212/WNL.0000000000012451
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., Cummings, J., & van der Flier, W. M. (2021). Alzheimer’s disease. Lancet, 397(10284), 1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Schneider, L. (2021). Cassava fraud and Alzheimer’s capitalism. Retrieved May 2022, from https://forbetterscience.com/2021/12/15/cassava-fraud-and-alzheimers-capitalism/
Servick, K. (2020). Biogen's Alzheimer's drug candidate takes a beating from FDA advisers. Retrieved May2022, from https://www.science.org/content/article/biogen-s-alzheimer-s-drug-candidate-takes-beating-fda-advisers
Sevigny, J., Chiao, P., Bussière, T., Weinreb, P. H., Williams, L., Maier, M., Dunstan, R., Salloway, S., Chen, T., Ling, Y., O’Gorman, J., Qian, F., Arastu, M., Li, M., Chollate, S., Brennan, M. S., Quintero-Monzon, O., Scannevin, R. H., Arnold, H. M., … Sandrock, A. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature, 537(7618), 50–56. https://doi.org/10.1038/nature19323
Siemers, E. R., Sundell, K. L., Carlson, C., Case, M., Sethuraman, G., Liu-Seifert, H., Dowsett, S. A., Pontecorvo, M. J., Dean, R. A., & Demattos, R. (2016). Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimer’s and Dementia, 12(2), 110–120. https://doi.org/10.1016/j.jalz.2015.06.1893
Syed, Y. Y. (2020). Sodium oligomannate: First approval. Drugs, 80(4), 441–444. https://doi.org/10.1007/s40265-020-01268-1
Tampi, R. R., Forester, B. P., & Agronin, M. (2021). Aducanumab: Evidence from clinical trial data and controversies. Drugs Context. https://doi.org/10.7573/dic.2021-7-3
Turner, E. H., Matthews, A. M., Linardatos, E., Tell, R. A., & Rosenthal, R. (2008). Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine, 358(3), 252–260. https://doi.org/10.1056/NEJMsa065779
Walsh, S., Merrick, R., Milne, R., & Brayne, C. (2021). Aducanumab for Alzheimer’s disease? BMJ, 374, n1682. https://doi.org/10.1136/bmj.n1682
Wang, T., Kuang, W., Chen, W., Xu, W., Zhang, L., Li, Y., et al. (2020a). A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimer’s Research & Therapy, 12(1), 110. https://doi.org/10.1186/s13195-020-00678-3
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., Xie, Z., Chu, X., Yang, J., Wang, H., Chang, S., Gong, Y., Ruan, L., Zhang, G., Yan, S., Lian, W., Du, C., Yang, D., Zhang, Q., … Geng, M. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Research, 29(10), 787–803. https://doi.org/10.1038/s41422-019-0216-x
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., Xie, Z., Chu, X., Yang, J., Wang, H., Chang, S., Gong, Y., Ruan, L., Zhang, G., Yan, S., Lian, W., Du, C., Yang, D., Zhang, Q., … Geng, M. (2020b). Geng et al. reply. Cell Research, 30(9), 820. https://doi.org/10.1038/s41422-020-0377-7
Willyard, C. (2021). How gut microbes could drive brain disorders. Nature, 590(7844), 22–25. https://doi.org/10.1038/d41586-021-00260-3
Xiao, S., Chan, P., Wang, T., Hong, Z., Wang, S., Kuang, W., et al. (2021). A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimer’s Research & Therapy, 13(1), 62. https://doi.org/10.1186/s13195-021-00795-7
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial or other conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeo-Teh, N.S.L., Tang, B.L. A Review of Scientific Ethics Issues Associated with the Recently Approved Drugs for Alzheimer’s Disease. Sci Eng Ethics 29, 2 (2023). https://doi.org/10.1007/s11948-022-00422-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11948-022-00422-0