Abstract
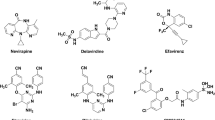
Quantitative structure-activity relationships (QSAR) and Comparative Molecular Field Analysis (CoMFA) have been applied in order to explain the structural requirements of HIV-1 reverse transcriptase (HIV-1 RT) inhibitory activity of TIBO derivatives on the MT-4 cells. The best QSAR model is satisfactory in both statistical significance and predictive ability. The derived structural descriptors indicate the importance of electronic contributions toward the HIV-1 RT inhibition of this class of compounds. However, it could not reveal any hydrophobic influence because of high collinearity between C2 and log P variables. In order to cope with steric interaction in the correlation, 3D-QSAR was performed using CoMFA. The obtained CoMFA model shows high predictive ability, r2 cv=0.771, and clearly demonstrates its potential in the steric feature of the molecules through contour maps, explaining a majority (81.8%) of the variance in the data. Consequently, these results can be useful in identifying the structural requirements of TIBO derivatives and helpful for better understanding the HIV-1 RT inhibition. Eventually, they provide a beneficial basis to design new and more potent inhibitors of HIV-1 RT.
Similar content being viewed by others
References
Pauwels, R., Andries, K., Desmyter, J., Schols, D., Kukla, M.J., Breslin, H.J., Raeymaechers, A., Gelder, J.V., Woestenborghs, R., Heykants, J., Schellekens, K., Janessen, M.A.C., De Clercq, E. and Janssen, P.A.J., Nature, 343 (1990) 470.
Frank, K.B., Noll, G.J., Connell, E.V. and Sim, I.S., J. Biol. Chem., 266 (1991) 14232.
Althaus, I.W., Chou, J.J., Gonzales, A.J., Deibel, M.R., Chou, K.-C., Kezdy, F.J., Romero, D.L., Aristoff, P.A., Tarpley, W.G. and Reusser, F., J. Biol. Chem., 268 (1993) 6119.
Spence, R.A., Kati, W.M., Anderson, K.S. and Johnson, K.A., Science, 267 (1995) 988.
Kohlstaedt, L.A., Wang, J., Friedman, J.M., Rice, P.A. and Steitz, T.A., Science, 256 (1992) 1783.
Ding, J., Das, K., Tantillo, C., Zhang, W., Clark, A.D.J., Jessen, S., Lu, X., Hsiou, Y., Jacobo-Molina, A., Andries, K., Pauwels, R., Moereels, H., Koymans, L, Janssen, P.A.J., Smith, R.H.J., Kroeger Koepke, R., Michejda, C.J., Hughes, S.H. and Arnold, E., Structure, 3 (1995) 365.
Ren, J.S., Esnouf, R., Hopkins, A., Ross, C., Jones, Y., Stammers, D. and Stuart, D., Structure, 3 (1995) 915.
Richman, D., Shih, C.-K., Lowy, I., Rose, J., Prodanovich, P., Goff, S. and Griffin, J., Proc. Natl. Acad. Sci. USA, 88 (1991) 11241.
Das, K., Ding, J., Hsiou, Y., Clark Jr., A.D., Moereels, H., Koymans, L., Andries, K., Pauwels, R., Janssen, R.A.J., Boyer, P.L., Clark, P., Smith Jr., R.H., Smith, M.B.K., Michejda, C.J., Hughes, S.H. and Arnold, E., J. Mol. Biol., 264 (1996) 1085.
Tantillo, C., Ding, J., Jacobo-Molina, A., Nanni, R.G., Boyer, P.L., Hughes, S.H., Pauwels, R., Andries, K., Janssen, P.A.J. and Arnold, E., J. Mol. Biol., 243 (1994) 369.
Hannongbua, S., Lawtrakul, L. and Limtrakul, J., J. Comput.-Aided Mol. Design, 10 (1996) 145.
Hannongbua, S., Lawtrakul, L., Sotriffer, C.A. and Rode, B.M., Quant. Struct.-Act. Relat., 15 (1996) 389.
Hansch, C. and Fujita, T., J. Am. Chem. Soc., 86 (1964) 1616.
Cramer III, R.D., Patterson, D.E. and Bunce, J.D., J. Am. Chem. Soc., 110 (1988) 5959.
Breslin, H.J., Kukla, M.J., Ludovici, D.W., Mohrbacher, R., Ho, W., Miranda, M., Rodgers, J.D., Hitchens, T.K., Leo, G., Gauthier, D.A., Ho, C.Y., Scott, M.K., De Clercq, E., Pauwels, R., Andries, K., Janssen, M.A.C. and Janssen, P.A.J., J. Med. Chem., 38 (1995) 771.
Tripos Associates Inc., St. Louis, MO.
Dewar, M.J., Zoebisch, E.G., Healy, E.F. and Stewart, J.P., J. Am. Chem. Soc., 107 (1985) 3902.
GAUSSIAN 94, Pittsburgh, PA, 1995.
ChemPlus 1.0, Hypercube Inc., Waterloo, ON, USA, 1993.
Miller, K.J., J. Am. Chem. Soc., 112 (1990) 8533.
Norusis, M.J., SPSS for Windows Release 6.0., SPSS, Inc., Chicago, 1993.
Quantum Chemistry Program Exchange, Indiana University, Bloomington, IN.
SYBYL Molecular Modelling Software, version 6.4, Tripos Associates, Inc., St. Louis, MO. 1996.
Wold, S., Johansson, E. and Cocchi, M., In Kubinyi, H. (Ed.) 3D QSAR in Drug Design: Theory, Methods and Applications, ESCOM, Leiden, 1993, pp. 523-549.
SYBYL Molecular Modelling Software, version 6.3, SYBYL Ligand Base Design, Tripos Associates, Inc., St. Louis, MO, 1996, p. 229.
Topliss, J.G. and Costello, R.J., J. Med. Chem., 15 (1972) 1066.
Wold, S., Quant. Struct.-Act. Relat., 10 (1991) 191.
Ho, W., Kukla, M.J., Breslin, H.J., Lodevici, D.W., Grows, P.P., Diamond, C.J., Miranda, M., Rodgers, D.W., Ho, C.Y., De Clercq, E., Pauwels, R., Andries, K., Janssen, M.A.C. and Janssen, P.A.J., J. Med. Chem., 38 (1995) 794.
Kukla, M.J., Breslin, H.J., Diamond, C.J., Grods, P.P., Ho, C.Y., Miranda, M., Roders, J.D., Sherril, R.G., De Clercq, E. and Janssen, P.A.J., J. Med. Chem., 34 (1991) 3187.
Kukla, M.J., Breslin, H.J., Pauwels, R., Fedde, C.L., Scott, M.K., Miranda, M., Sherril, R.G., Raeymaeker, A., Gelder, J.V., Andries, K., Janssen, M.A.C., De Clercq, E. and Janssen, P.A.J., J. Med. Chem., 34 (1991) 746.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology, Wiley Interscience, New York, NY, 1979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hannongbua, S., Pungpo, P., Limtrakul, J. et al. Quantitative structure-activity relationships and comparative molecular field analysis of TIBO derivatised HIV-1 reverse transcriptase inhibitors. J Comput Aided Mol Des 13, 563–577 (1999). https://doi.org/10.1023/A:1008013917905
Issue Date:
DOI: https://doi.org/10.1023/A:1008013917905