Abstract
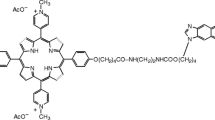
On the basis of theoretical computations, we have recently synthesised [Perrée-Fauvet, M. and Gresh, N., Tetrahedron Lett., 36 (1995) 4227] a bisarginyl conjugate of a tricationic porphyrin (BAP), designed to target, in the major groove of DNA, the d(GGC GCC)2 sequence which is part of the primary binding site of the HIV-1 retrovirus site [Wain-Hobson, S. et al., Cell, 40 (1985) 9]. In the theoretical model, the chromophore intercalates at the central d(CpG)2 step and each of the arginyl arms targets O6/N7belonging to guanine bases flanking the intercalation site. Recent IR and UV-visible spectroscopic studies have confirmed the essential features of these theoretical predictions [Mohammadi, S. et al., Biochemistry, 37 (1998) 6165]. In the present study, we compare the energies of competing intercalation modes of BAP to several double-stranded oligonucleotides, according to whether one, two or three N- methylpyridinium rings project into the major groove. Correspondingly, three minor groove binding modes were considered, the arginyl arms now targeting N3, O2 sites belonging to the purine or pyrimidine bases flanking the intercalation site. This investigation has shown that: (i) in both the major and minor grooves, the best-bound complexes have the three N-methylpyridinium rings in the groove opposite to that of the phenyl group bearing the arginyl arms; (ii) major groove binding is preferred over minor groove binding by a significant energy (29 kcal/mol); and (iii) the best-bound sequence in the major groove is d(GGC GCC)2 with two successive guanines upstream from the intercalation. On the other hand, due to the flexibility of the arginyl arms, other GC-rich sequences have close binding energies, two of them being less stable than it by less than 8 kcal/mol. These results serve as the basis for the design of derivatives of BAP with enhanced sequence selectivities in the major groove.
Similar content being viewed by others
References
Steitz, T.A., Q. Rev. Biophys., 23 (1990) 205.
Brennan, R.G., Curr. Opin. Struct. Biol., 2 (1992) 100.
Harrison, S., Nature, 353 (1991) 715.
Seeman, N., Rosenberg, J. and Rich, A., Proc. Natl. Acad. Sci. USA, 73 (1976) 804.
Hélène, C., FEBS Lett., 74 (1977) 10.
Pavletich, N.P. and Pabo, C.O., Science, 252 (1991) 809.
Pavletich, N.P. and Pabo, C.O., Science, 261 (1993) 1701.
Marmorstein, R., Carey, M., Ptashne, M. and Harrison, S.C., Nature, 356 (1992) 408.
Konig, P., Giraldo, R., Chapman, L. and Rhodes, D., Cell, 85 (1996) 125.
Baleja, J.D., Marmorstein, R., Harrison, S.C. and Wagner, G., Nature, 356 (1992) 450.
Desjarlais, J.R. and Berg, J.M., Proteins Struct. Funct. Genet., 12 (1992) 101.
Desjarlais, J.R. and Berg, J.M., Proc. Natl. Acad. Sci. USA, 89 (1992) 7345.
Choo, Y. and Klug, A., Proc. Natl. Acad. Sci. USA, 91 (1994) 11163 and 11168.
Jamieson, A.C., Kim, S.-H. and Wells, J.A., Biochemistry, 33 (1994) 5689.
Lustig, M. and Jernigan, R.L., Nucleic Acids Res., 23 (1995) 4707.
Rebar, E.J. and Pabo, C.O., Science, 263 (1994) 671.
Suzuki, M., Gerstein, M. and Yagi, N., Nucleic Acids Res., 22 (1994) 3397.
Wu, H., Yang, W.R. and Barbas III, C.F., Proc. Natl. Acad. Sci. USA, 92 (1995) 344.
Gresh, N. and Kahn, P.H., J. Biomol. Struct. Dyn., 7 (1990) 1141.
Gresh, N. and Kahn, P.H., J. Biomol. Struct. Dyn., 8 (1991) 827.
Gresh, N., René, B., Hui, X., Barsi, M.-C., Roques, B.P. and Garbay, C., J. Biomol. Struct. Dyn., 12 (1994) 91.
Perrée-Fauvet, M. and Gresh, N., Tetrahedron Lett., 36 (1995) 4227.
Gresh, N., J. Biomol. Struct. Dyn., 14 (1996) 255.
Tsimanis, A., Bichko, V., Dreilina, D., Meldrais, J., Lozha, V., Kukaine, R. and Gren, E., Nucleic Acids Res., 11 (1983) 6079.
Hong, F.D., Huang, H.-J.S., To, H., Young, L.-J.S., Oro, A., Bookstein, R., Lee, E.Y.-H.P. and Lee, W.-H., Proc. Natl. Acad. Sci. USA, 86 (1989) 5502.
Dvorak, M., Urbanek, P., Bartunek, P., Paces, V., Vlach, J., Pecenka, V., Arnold, L., Travnicek, M. and Riman, J., Nucleic Acids Res., 17 (1989) 5651.
Smith, S., Baker, D. and Jardines, L., Biochem. Biophys. Res. Commun., 160 (1989) 1397.
Timsit, Y. and Moras, D., J. Mol. Biol., 251 (1995) 629.
Wain-Hobson, S., Sonigo, P., Danos, O., Cole, S. and Alizon, M., Cell, 40 (1985) 9.
Ratner, L., Haseltine, W., Patarca, R., Livak, K.J., Starcich, B., Josephs, S.F., Doran, E.R., Rafalski, J.A., Whitehorn, E.A., Baumeister, K., Ivanoff, L., Petteway Jr., S.R., Peaerson, M.L., Lautenberger, J.A., Papas, T.S., Ghrayeb, J., Chang, N.T., Gallo, R.C. and Wong-Staal, F., Nature, 313 (1985) 277.
Mohammadi, S., Perrée-Fauvet, M., Gresh, N., Hillairet, K. and Taillandier, E., Biochemistry, 37 (1998) 6165.
Fiel, R.J., Howard, J.C., Mark, E.H. and Datta Gupta, N., Nucleic Acids Res., 6 (1979) 3093.
Pasternack, R.F. and Gibbs, E.J., In Tullius, T. (Ed.) Metal DNA Chemistry, American Chemical Society, Washington, DC, 1989, pp. 59–73.
Pasternack, R.F. and Gibbs, E.J., In Sigel, A. and Sigel, H. (Eds.) Metal Ions in Biological Systems, Vol. 33, Marcel Dekker, New York, NY, 1996, pp. 367–397.
Moser, H.E. and Dervan, P.B., Science, 238 (1987) 645.
Le Doan, T., Perrouault, L., Praseuth, D., Habhoub, N., Decout, J.-L., Thuong, N.T., Lhomme, J. and Hélène, C., Nucleic Acids Res., 15 (1987) 7749.
Hélène, C. and Toulmé, J.J., Biochim. Biophys. Acta, 1049 (1990) 99.
De Mesmaeker, A., Häner, R., Martin, P. and Moser, H.E., Acc. Chem. Res., 28 (1995) 366.
Escudé, C., Nguyen, C.H., Mergny, J.L., Sun, J.S., Bisagni, E., Garestier, T. and Hélène, C., J. Am. Chem. Soc., 117 (1995) 10212.
Hyrup, B. and Nielsen, P.E., Bioorg. Med. Chem., 4 (1996) 5.
Park, C., Campbell, J.L. and Goddard III, W.A., J. Am. Chem. Soc., 117 (1995) 6287.
Cuenoud, B. and Schepartz, A., Science, 259 (1993) 510.
Terbrueggen, R.H. and Barton, J.K., Biochemistry, 34 (1995) 8227.
Arcamone, F., Doxorubicin, Anticancer Antibiotics, Academic Press, New York, NY, 1981.
Lee, S.H. and Goldberg, I.H., Biochemistry, 28 (1989) 1019.
Zein, N., Poncin, M., Nilakantan, R. and Ellestad, G.A., Science, 244 (1989) 697.
Ho, S.N., Boyer, S.H., Schreiber, S.L., Danishefshky, S.J. and Crabtree, G.R., Proc. Natl. Acad. Sci. USA, 91 (1994) 9203.
Zimmer, C. and Wahnert, U., Prog. Biophys. Mol. Biol., 41 (1986) 31.
Mrksich, M., Wade, W.S., Dwyer, T.J., Geierstanger, B.H., Wemmer, D.E. and Dervan, P.B., Proc. Natl. Acad. Sci. USA, 89 (1992) 7586.
Dwyer, T.J., Geierstanger, B.H., Bathini, Y., Lown, J.W. and Wemmer, D.E., J. Am. Chem. Soc., 114 (1992) 5911.
Nikolaev, V.A., Grokhovsky, S.L., Surovaya, A.N. and Gursky, G.V., J. Biomol. Struct. Dyn., 14 (1996) 31.
Bailly, C., Helbecque, N., Hénichart, J.-P., Colson, P., Houssier, C., Rao, K.E., Shea, R.G. and Lown, J.W., J. Mol. Recog., 3 (1990) 26.
Bailly, C. and Hénichart, J.-P., Bioconj. Chem., 2 (1991) 379.
Anneheim-Herbelin, G., Perrée-Fauvet, M., Gaudemer, A., Hélissey, P., Giorgi-Renault, S. and Gresh, N., Tetrahedron Lett., 34 (1993) 7263.
Goulaouic, H., Carteau, S., Subra, F., Mouscadet, J.-F., Auclair, C. and Sun, J.-S., Biochemistry, 33 (1994) 1412.
Bourdouxhe-Housiaux, C., Colson, P., Houssier, C., Waring, M.J. and Bailly, C., Biochemistry, 35 (1996) 4251.
Hélissey, P., Bailly, C., Vishwakarma, J.N., Auclair, C., Waring, M.J. and Giorgi-Renault, S., Anti-Cancer Drug Des., 11 (1996) 527.
Hui, X. and Gresh, N., J. Biomol. Struct. Dyn., 11 (1993) 333.
Perrée-Fauvet, M. and Gresh, N., J. Biomol. Struct. Dyn., 11 (1994) 1203.
Lavery, R., In Wells, R.D. and Harvey, S.C. (Eds.), Unusual DNA Structures, Springer, New York, NY, 1988, pp. 189–206.
Lavery, R., Adv. Comput. Biol., 1 (1994) 69.
Pullman, B. and Pullman, A., Q. Rev. Biophys., 14 (1981) 289.
Flatters, D., Zakrzewska, K. and Lavery, R., J. Comput. Chem., 18 (1997) 1043.
Jones, K.A., Kadonaga, J.T., Luciw, P.A. and Tjian, R., Science, 232 (1986) 755.
Murdock, K.C., Child, R.C., Fabio, P.F., Angier, R.B., Wallace, R.E., Durr, F.E. and Citarella, R.V., J. Med. Chem., 22 (1979) 1024.
Wallace, R.E., Murdock, K.C., Angier, R.B. and Durr, F.E., Cancer Res., 39 (1979) 1570.
Garbay-Jaureguiberry, C., Esnault, C., Delepierre, M., Laugaa, P., Laalami, S., Le Pecq, J.-B. and Roques, B.P., Drugs Exp. Clin. Res., XIII (1987) 353.
Garbay-Jaureguiberry, C., Barsi, M.-C., Jacquemin-Sablon, A., Le Pecq, J.-B. and Roques, B. P., J.Med. Chem., 35 (1992) 72.
Langlet, J., Claverie, P., Caillet, J. and Pullman, A., J. Phys. Chem., 92 (1988) 1631.
Langlet, J., Gresh, N. and Giessner-Prettre, C., Biopolymers, 36 (1995) 765.
Gresh, N. and Roques, B.P., Biopolymers, 47 (1997) 145.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gresh, N., Perrée-fauvet, M. Major versus minor groove DNA binding of a bisarginylporphyrin hybrid molecule: A molecular mechanics investigation. J Comput Aided Mol Des 13, 123–137 (1999). https://doi.org/10.1023/A:1008033219724
Issue Date:
DOI: https://doi.org/10.1023/A:1008033219724