Abstract
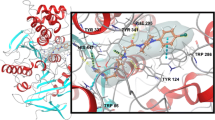
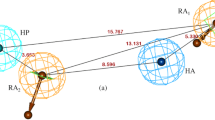
Automated docking and three-dimensional Quantitative Structure-Activity Relationship studies (3D QSAR) were performed for a series of 82 reversible, competitive and selective acetylcholinesterase (AChE) inhibitors. The suggested automated docking technique, making use of constraints taken from experimental crystallographic data, allowed to dock all the 82 substituted N-benzylpiperidines to the crystal structure of mouse AChE, because of short computational times. A 3D QSAR model was then established using the CoMFA method. In contrast to conventional CoMFA studies, the compounds were not fitted to a reference molecule but taken in their 'natural' alignment obtained by the docking study. The established and validated CoMFA model was then applied to another series of 29 N-benzylpiperidine derivatives whose AChE inhibitory activity data were measured under different experimental conditions. A good correlation between predicted and experimental activity data shows that the model can be extended to AChE inhibitory activity data measured on another acetylcholinesterase and/or at different incubation times and pH level.
Similar content being viewed by others
References
Rosser, M.N., Iversen, L.L., Reynolds, G.P., Mountjoy, C.A. and Roth, M., Br. Med. J., 288 (1984) 961.
Barnard, E.A., In Hubbard, J.I. (Ed.) The Peripheral Nervous System, Plenum, New York, NY, 1974, pp. 201–224.
Sims, N.R., Bowen, D.M., Allen, S.J., Smith, C.C.T., Neary, D., Thomas, D.J. and Davison, A.N., J. Neurochem., 40 (1983) 503.
Perry, E.K., Br. Med. Bull., 42 (1986) 408.
Becker, R.E. and Giacobini, E., Drug Dev. Res., 12 (1988) 163.
Johns, C.A., Haroutunian, V., Greenwald, B.S., Mohs, R.C., Davis, B.M., Kanof, P., Horvath, T.B. and Davis, K.L., Drug. Dev. Res., 5 (1985) 77.
Brennan, M.B., Chem. Eng. News, 20 (1997) 29.
John, V., Lieberburg, I. and Thorsett, E.D., Annu. Rep. Med. Chem., 28 (1993) 197.
Sugimoto, H., Tsuchiya, T., Sugumi, H., Higurashi, K., Karibe, N., Iimura, Y., Sasaki, A., Kawakami, Y., Nakamura, T., Araki, S., Yamanishi, Y. and Yamatsu, K., J. Med. Chem., 33 (1990) 1880.
Sugimoto, H., Tsuchiya, T., Sugumi, H., Higurashi, K., Karibe, N., Iimura, Y., Sasaki, A., Kawakami, Y., Araki, S., Yamanishi, Y. and Yamatsu, K., J. Med. Chem., 35 (1992) 4542.
Pang, Y.-P. and Kozikowski, A.P., J. Comput.-Aided Mol. Design, 8 (1994) 683.
Yamamoto, Y., Ishihara, Y. and Kuntz, I.D., J. Med. Chem., 37 (1994) 3141.
Inoue, A., Kawai, T., Wakita, M., Iimura, Y., Sugimoto, H. and Kawakan, Y., J. Med. Chem., 39 (1996) 4460.
Villalobos, A., Blake, J.F., Biggers, C.K., Butler, T.W., Chapin, D.S., Chen, Y.L., Ives, J.L., Jones, S.B., Liston, D.R., Nagel, A.A., Nason, D.M., Nielsen, J.A., Shalaby, I.A. and Frost White, W., J. Med. Chem., 37 (1994) 2721.
Tong, W., Collantes, E.R., Chen, Y. and Welsh, W.J., J. Med. Chem., 39 (1996) 380.
Cho, S.J., Serrano Garsia, M.L., Bier, J. and Tropsha, A., J. Med. Chem., 39 (1996) 5064.
Cardozo, M.G., Imura, Y., Sugimoto, H., Yamanishi, Y. and Hopfinger, A.J., J. Med. Chem., 35 (1992) 584.
Kuntz, I.D., Meng, E.C. and Shoichet, B.K., Acc. Chem. Res., 27 (1994) 117.
Cramer III, R.D., Patterson, D.E. and Bunce, J.D., J. Am. Chem. Soc., 110 (1988) 5959.
Waller, C.L., Oprea, T.I., Giolitti, A. and Marshall, G.R., J. Med. Chem., 36 (1993) 4152.
The program Sybyl 6.3 is available from Tripos Associates, St. Louis, MO.
Weiner, S.J., Kollman, P.A., Nguyen, D.T. and Case, D.A., J. Comput. Chem., 7 (1986) 230.
Harel, M., Schalk, I., Ehret-Sabatier, L., Bouet, F., Goeldner, M., Hirth, C., Axelsen, P., Silman, I. and Sussman, J. L., Proc. Natl. Acad. Sci. USA, 90 (1993) 9031.
Dougherty, D.A., Science, 271 (1996) 163.
Clarc, M., Cramer III, R.D. and Van Opdenbosch, N., J. Comput. Chem., 10 (1989) 982.
Villalobos, A., Butler, T.W., Chapin, D.S., Chen, Y.L., DeMattos, S.B., Ives, J.L., Jones, S.B., Liston, D.R., Nagel, A.A., Nason, D.M., Nielsen, J.A., Ramirez, A.D., Shalaby, I.A. and Frost White, W., J. Med. Chem., 38 (1995) 2802.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
Cramer, R.D., Bunce, J.D., Patterson, D.E. and Frank, I.E., Quant. Struct.-Act. Relat., 7 (1988) 18.
Sussman, J.L., Harel, M., Frolow, F., Oefner, C., Goldman, A., Toker, L. and Silman, I., Science, 253 (1991) 872.
Ordentlich, A., Barak, D., Kronman, Ch., Flashner, Y., Leitner, M., Segal, Y., Ariel, N., Cohen, S., Velan, B. and Shafferman, A., J. Biol. Chem., 268 (1993) 17083.
Radic, Z., Pickering, N.A., Vellom, D.C., Camp, Sh. and Taylor, P., Biochemistry, 32 (1993) 12074.
Ishihara, Y., Kato, K. and Goto, G., Chem. Pharm. Bull., 39 (1991) 3225.
Fink, D.M., Bores, G.M., Effland, R.C., Huger, F.P., Kurys, B.E., Rush, D.K. and Selk, D.E., J. Med. Chem., 38 (1995) 3645.
Clementi, S. and Wold, S., In Van de Waterbeemd, H. (Ed.) Chemometric Methods in Drug Design, VCH, Weinheim, 1995, pp. 49–62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernard, P., Kireev, D.B., Chrétien, J.R. et al. Automated docking of 82 N-benzylpiperidine derivatives to mouse acetylcholinesterase and comparative molecular field analysis with 'natural' alignment. J Comput Aided Mol Des 13, 355–371 (1999). https://doi.org/10.1023/A:1008071118697
Issue Date:
DOI: https://doi.org/10.1023/A:1008071118697