Abstract
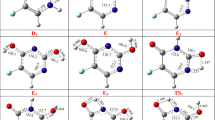
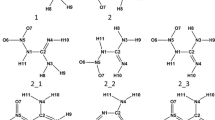
Ab initio geometry optimizations were carried out at the HF/3-21G and HF/6-31+G** levels for the six tautomeric forms of 2-thiouracil (2TU, 2TU1, 2TU2, 2TU3, 2TU4, 2TU5) in the gas phase and in solution. To obtain a more definitive estimate of the relative stabilities for 2-thiouracil tautomers in the gas phase, single-point MP2/6-31+G** calculations were performed on the HF/6-31+G** optimized geometries. The tautomeric equilibria in 1,4-dioxane (ε=2.21), acetonitrile (ε=38), and in water (ε=78.54) were studied using the self-consistent reaction field (SCRF) theory. The calculated relative free energies indicated that 2TU is the energetically preferred tautomer in the gas phase and in solution. The stability order of 2-thiouracil tautomers depends on the level of theory and the dielectric constant of the solvent. The obtained results are compared with the available experimental data.
Similar content being viewed by others
References
Thewald, U. and Bugg, C.E., J. Am. Chem. Soc., 94 (1972) 8892.
Saenger, W. and Suck, D., Eur. J. Biochem., 82 (1973) 473.
Gottschalk, E., Kopp, E. and Lezius, A.G., Eur. J. Biochem., 24 (1971) 168.
Löwdin, P.O., Adv. Quantum Chem., 2 (1965) 213.
Winckelmann, I. and Larsen, E.H., Synthesis, (1986) 1041.
Ghomi, M., Letellier, R., Taillandier, E., Chinsky, L., Laigle, A. and Turpin, P.Y., J. Raman Spectrosc., 17 (1986) 249.
Leś, A. and Adamowicz, L., J. Am. Chem. Soc., 112 (1990) 1504.
Katritzky, A.R., Szafran, M. and Stevens, J., J. Chem. Soc. Perkin Trans. II, (1989) 1507.
Leś, A. and Ortega-Blake, I., Int. J. Quantum Chem., 30 (1986) 225.
Aruna, S. and Shanmugan, G., Indian J. Chem., 25A (1986) 256.
Rostkowska, H., Barski, A., Szczepaniak, K., Szczesniak, M. and Person, W.B., J. Mol. Struct., 176 (1988) 137.
Rostkowska, H., Szczepaniak, K., Nowak, M.J., Leszczynski, J., KuBulat, K. and Person, W.B., J. Am. Chem. Soc., 112 (1990) 2147.
Psoda, A. and Shugar, D., Acta Biochim. Pol., 26 (1979) 55.
Okabe, N., Fujiwara, T., Yamagata, Y. and Tomita, T., Bull. Chem. Soc. Jpn., 56 (1983) 1543.
Katritzky, A.R., Baykut, G., Rachwal, S., Szafran, M., Caster, K.C. and Eyler, J., J. Chem. Soc. Perkin Trans. II, (1989) 1499.
Karelson, M.M., Katritzky, A.R., Szafran, M. and Zerner, M.C., J. Org. Chem., 54 (1989) 6030.
Wong, M.W., Leung-Toung, R. and Wentrup, C., J. Am. Chem. Soc., 115 (1993) 2465.
Parchment, O.G., Green, D.V.S., Taylor, P.J. and Hillier, I.H., J. Am. Chem. Soc., 115 (1993) 2352.
Cieplak, P., Bash, P., Singh, U.C. and Kollman, P.A., J. Am. Chem. Soc., 109 (1987) 6283.
Cao, M., Teppen, B.J., Miller, D.M., Pranata, J. and Schäfer, L., J. Phys. Chem., 98 (1994) 11353.
Cramer, C.J. and Truhlar, D.G., J. Am. Chem. Soc., 115 (1993) 8810.
Orozco, M. and Luque, F.J., Biopolymers, 33 (1993) 1851.
Kwiatkowski, J.S., Zielinski, T.J. and Rein, R., Adv. Quantum Chem., 18 (1986) 85.
Gould, I.R. and Hillier, I.H., Chem. Phys. Lett., 161 (1989) 185.
Kwiatkowski, J.S., Bartlett, R.J. and Person, W.B., J. Am. Chem. Soc., 110 (1988) 2353.
Leś, A. and Adamowicz, L., J. Phys. Chem., 93 (1989) 7078.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Robb, M.A., Cheeseman, J.R., Keith, T.A., Petersson, G.A., Montgomery, J.A., Raghavachari, K., Al-Laham, M.A., Zakrzewski, V.G., Ortiz, J.V., Foresman, J.B., Cioslowski, J., Stefanov, B.B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y. and Chen, W., GAUSSIAN 94, Revision B.3., Gaussian Inc., Pittsburgh, PA, 1995.
Onsager, L., J. Am. Chem. Soc., 58 (1936) 1486.
Wong, M.W., Frisch, M.J. and Wiberg, K.B., J. Am. Chem. Soc., 113 (1991) 4776.
Delage, C., H'Naifi, A., Goursolle, M. and Carpy, A., C. R. Seances Acad. Sci. Paris, Serie II, 302 (1986) 219.
ChemOffice Pro for Windows, Cambridge Scientific Computing Inc., Cambridge, MA.
Hawkinson, S.W., Acta Crystallogr., B33 (1977) 80.
Lesyng, B. and Saenger, W.Z., Naturforsch., C: Biosci., 36 (1981) 956.
Leszczyński, J. and Lammertsma, K., J. Phys. Chem., 95 (1991) 3128.
Psoda, A., Kazimierczuk, Z. and Shugar, D., J. Am. Chem. Soc., 96 (1974) 6832.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yekeler, H. Ab initio study on tautomerism of 2-thiouracil in the gas phase and in solution. J Comput Aided Mol Des 14, 243–250 (2000). https://doi.org/10.1023/A:1008132202838
Issue Date:
DOI: https://doi.org/10.1023/A:1008132202838