Abstract
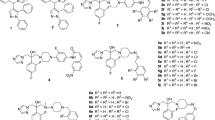
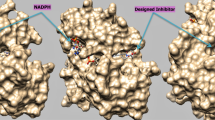
A series of 5-(arylthio)-2,4-diaminoquinazolines are known as selective inhibitors of dihydrofolate reductase (DHFR) from Candida albicans. We have performed docking and molecular dynamics simulations of these inhibitors with C. albicans and human DHFR to understand the basis for selectivity of these agents. Study was performed on a selected set of 10 compounds with variation in structure and activity. Molecular dynamics simulations were performed at 300 K for 45 ps with equilibration for 10 ps. Trajectory data was analyzed on the basis of hydrogen bond interactions, energy of binding and conformational energy difference. The results indicate that hydrogen bonds formed between the compound and the active site residues are responsible for inhibition and higher potency. The selectivity index, i.e the ratio of I50 against human DHFR to I50 against fungal DHFR, is mainly determined by the conformation adapted by the compounds within the active site of two enzymes. Since the human DHFR active site is rigid, the compound is trapped in a higher energy conformation. This energy difference between the two conformations ΔE mainly governs the selectivity against fungal DHFR. The information generated from this analysis of potency and selectivity should be useful for further work in the area of antifungal research.
Similar content being viewed by others
References
Kuyper, L.F., In Perun, T.J. and Propst, C.L. (Eds.) Computer-Aided Drug Design Methods and Applications, Marcel Dekker Inc., New York, NY and Basel, 1989, pp. 327-369.
McCormack, J., In Hansch, C. and Sammes, P.G. (Eds.) Comprehensive Medicinal Chemistry, Vol. 2., Pergamon Press, London, 1990, pp. 271-298.
Volz, K.W., Matthews, D.A., Alden, R.A., Freer, S.T., Hansch, C., Kaufman, B.T. and Kraut, J., J. Biol. Chem., 257 (1982) 2528.
Matthews, D.A., Alden, R.A., Bolin, J.T., Filman, D.J., Freer, S.T., Hamlin, R., Hol, W.G.J., Kisluik, R.L., Pastore, E.J., Plante, L.T., Xuong, N. and Kraut, J., J. Biol. Chem., 253 (1978) 6946.
Bolin, J.T., Filman, D.J., Matthews, D.A., Hamlin, R.C. and Kraut, J., J. Biol. Chem., 257 (1982) 13650.
Whitlow, M., Howard, A.J., Stewart, D., Hardman, K.D., Kuyper, L.F., Baccanari, D.P., Fling, M.E. and Tansik, R.L., J. Biol. Chem., 272 (1997) 30289.
Chan, J.H., Hong, J.S., Kuyper, L.F., Baccanari, D.P., Joyner, S.S., Tansik, R.L., Boytos, C.M. and Rudolph, S.K., J. Med. Chem., 38 (1995) 3608.
Kuyper, L.F., Baccanari, D.P., Jones, M.L., Hunter, R.N., Tansik, R.L., Joyner, S.S., Boytos, C.M., Rudolph, S.K., Knick, V., Robert Wilson, H., Marc Caddell, J., Friedman, H.S., Comley, J.C.W. and Stables, J.N., J. Med. Chem., 39 (1996) 892.
Inoue, A., Kawai, T., Wakita, M., Iimura, Y., Sugimoto, H. and Kawakami, Y., J. Med. Chem., 39 (1996) 4460.
Yamamoto, Y., Ishihara, Y. and Kuntz, I.D., J. Med. Chem., 37 (1994) 3141.
Hariprasad, V. and Kulkarni, V.M., J. Mol. Model., 3 (1997) 443.
Rastelli, G. and Costantino, L., Bioorg. Med. Chem. Lett., 8 (1998) 641.
Kyle, D.J., Chakravarty, S., Sinsko, J.A. and Stormann, T.M., J. Med. Chem., 37 (1994) 1347.
Furet, P., Caravatti, G., Lydon, N., Priestle, J.P., Sowadski, J.M., Trinks, U. and Traxler, P., J. Comput.-Aided Mol. Design, 9 (1995) 465.
Winter, H.D. and Herdewijn, P., J. Med. Chem., 39 (1996) 4727.
Wang, S., Karanietz, M.G., Blumberg, P.M., Marquez, V.E. and Milne, G.W.A., J. Med. Chem., 39 (1996) 2541.
Insight II 97.0 Molecular Modeling software is available from Molecular Simulations Inc., San Diego, CA.
Discover 3.0.0 User Guide, October 1995, Molecular Simulations Inc. San Diego, CA.
Gilson, M.K. and Honig, B., Proteins, 4 (1988) 7.
Kulkarni, S.S. and Kulkarni, V.M., J. Chem. Inf. Comput. Sci., 39 (1999) 1128.
Hariprasad, V. and Kulkarni, V.M., J. Mol. Recogn., 9 (1996) 95.
Selassie, C.D., Gan, W., Kallander, L.S. and Klein, T.E., J. Med. Chem., 41 (1998) 4261.
Birdsall, B., Feeney, J., Tendler, S.J.B., Hammond, S.J. and Roberts, G.C.K., Biochemistry, 28 (1989) 2297.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gokhale, V.M., Kulkarni, V.M. Selectivity analysis of 5-(arylthio)-2,4-diaminoquinazolines as inhibitors of Candida albicans dihydrofolate reductase by molecular dynamics simulations. J Comput Aided Mol Des 14, 495–506 (2000). https://doi.org/10.1023/A:1008189724803
Issue Date:
DOI: https://doi.org/10.1023/A:1008189724803