Abstract
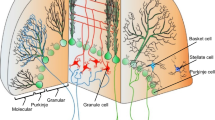
We present a simple method for the realistic description of neurons that is well suited to the development of large-scale neuronal network models where the interactions within and between neural circuits are the object of study rather than the details of dendritic signal propagation in individual cells. Referred to as the composite approach, it combines in a one-compartment model elements of both the leaky integrator cell and the conductance-based formalism of Hodgkin and Huxley (1952). Composite models treat the cell membrane as an equivalent circuit that contains ligand-gated synaptic, voltage-gated, and voltage- and concentration-dependent conductances. The time dependences of these various conductances are assumed to correlate with their spatial locations in the real cell. Thus, when viewed from the soma, ligand-gated synaptic and other dendritically located conductances can be modeled as either single alpha or double exponential functions of time, whereas, with the exception of discharge-related conductances, somatic and proximal dendritic conductances can be well approximated by simple current-voltage relationships. As an example of the composite approach to neuronal modeling we describe a composite model of a cerebellar Purkinje neuron.
Similar content being viewed by others
References
Barbour B (1993) Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 11:759-769.
Beyer WH (1966) Handbook of Tables for Probability and Statistics (2nd ed.). Chemical Rubber Co., Cleveland, OH.
Bhalla US, Iyengar R (1999) Emergent properties of networks of biological signalling pathways. Science 283:381-387.
Bush PC, Sejnowski TJ (1991) Simulations of a reconstructed cerebellar Purkinje cell based on simplified channel kinetics. Neural Comp. 3:321-332.
Chan CY, Hounsgaard J, Nicholson C (1988) Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells. J. Physiol. (Lond). 402:751-771.
Chang W, Strahlendorf JC, Strahlendorf HK (1993) Ionic contributions to the oscillatory firing activity of rat Purkinje cells in vitro. Brain Res. 614:335-341.
Coop AD, Reeke GN (2000) Simulating the temporal evolution of neuronal discharge. Neurocomputing. 32:91-96.
Crepel F, Penit-Soria J (1986) Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells: An in vitro study. J. Physiol. (Lond). 372:1-23.
Delcour AH, Lipscombe D, Tsien RW (1993) Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J. Neurosci. 13:181-194.
De Schutter E, Bower JM (1994) An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J. Neurophysiol. 71:375-400.
De Schutter E, Smolen P (1998) Calcium dynamics in large neuronal models. In: Koch C, Segev I. Methods in Neuronal Modeling (2nd ed). MIT Press, Cambridge, MA. pp. 211-250.
Fiala JC, Grossberg S, Bullock D (1996) Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. J. Neurosci. 16:3760-3774.
Gerstner W (1999) Spiking neurons. In: Maass, W, Bishop CM. Pulsed Neural Networks. MIT Press, Cambridge, MA.
Gruol DL, Jacquin T, Yool AJ (1991) Single-channel KC currents recorded from the somatic and dendritic regions of cerebellar Purkinje neurons in culture. J. Neurosci. 11:1002-1015.
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond). 117:500-544.
Holt GR, Koch C. (1999) Electrical interactions via the extracellular potential near cell bodies. J. Comp. Neurosci. 6:169-184.
Hounsgaard J, Midtgaard J (1988) Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. J. Physiol. (Lond). 402:731-749.
Hounsgaard J, Nicholson C (1983) Potassium accumulation around individual Purkinje cells in cerebellar slices from the guinea-pig. J. Physiol. (Lond). 340:359-388.
Jaeger D, Bower JM (1994) Prolonged response in rat cerebellar Purkinje cells following activation of the granule cell layer: An intracellular in vitro and in vivo investigation. Exp. Brain Res. 100:200-214.
Kay AR, Sugimori M, Llinás R (1998) Kinetic and stochastic properties of a persistent sodium current in mature guinea pig cerebellar Purkinje cells. J. Neurophysiol. 80:1167-1179.
Koch C, Segev I (1998) Methods in Neuronal Modeling (2nd ed). MIT Press, Cambridge, MA.
Latham PE, Richmond BJ, Nelson PG, Nirenberg S (2000) Intrinsic dynamics in neuronal networks. I. Theory. J. Neurophysiol. 83:808-827.
Llano I, DiPolo R, Marty A (1994) Calcium-induced calcium release in cerebellar Purkinje cells. Neuron 12:663-673.
Llano I, Marty A, Armstrong CM, Konnerth A (1991) Synaptic and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J. Physiol. (Lond). 434:183-213.
Llinás R, Sugimori M (1980a) Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J. Physiol. (Lond). 305:171-195.
Llinás R, Sugimori M (1980b) Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J. Physiol. (Lond). 305:197-213.
Lnenicka GA, Hong SJ (1997) Activity-dependent changes in voltage-dependent calcium currents and transmitter release. Mol. Neurobiol. 14:37-66.
Maass W, Bishop CM (1999) Pulsed Neural Networks. MIT Press, Cambridge, MA.
McCormick AA, Huguenard JR (1992) A model of the electrophysiological properties of thalamocortical relay neurons. J. Neurophysiol. 68:1384-1400.
Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD (2000) Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. J. Neurosci. 20:5516-5525.
Migliore M, Cook MEP, Jaffe DB, Turner DA, Johnston D (1995) Computer simulations of morphologically reconstructed CA3 hippocampal neurons. J. Neurophysiol. 73:1157-1168.
Neher E, Augustine GJ (1992) Calcium gradients and buffers in bovine chromaffin cells J. Physiol. (Lond). 450:273-301.
Pinsky PR, Rinzel J (1994) Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J. Comp. Neurosci. 1:39-60.
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1992) Numerical Recipes in C. Cambridge University Press, Cambridge.
Protopapas AD, Vanier M, Bower JM (1998) Simulating large networks of neurons. In: Koch C, Segev I. Methods in Neuronal Modeling (2nd ed). MIT Press, Cambridge, MA. pp. 461-498.
Rall W (1967) Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic inputs. J. Neurophysiol. 30:1138-1168.
Rall W, Burke RE, Holmes WR, Jack JJB, Redman SJ, Segev I (1992) Matching dendritic neuron models to experimental data. Physiol. Revs. 72:S159-S186.
Rapp M, Segev I, Yarom Y (1994) Physiology, morphology and detailed passive models of guinea-pig cerebellar Purkinje cells. J. Physiol. (Lond). 474:101-118.
Regan LJ (1991) Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J. Neurosci. 11:2259-2269.
Rudy B, Seeburg P, eds. (1999) Molecular and Functional Diversity of Ion Channels and Receptors. Anns. NY Acad. Sci. 868. NY Academy of Sciences, New York, NY.
Sah P (1996) Ca2+-activated K+ currents in neurons: Types, physiological roles and modulation. Trends Neurosci. 19:150-154.
Schweighofer N, Arbib MA (1998) A model of cerebellar metaplasticity. Learn. Mem. 4:421-428.
Schweighofer N, Arbib MA, Kawato M (1999) Role of the cerebellum in reaching movements in humans. II. A neural model of the intermediate cerebellum. Eur. J. Neurosci. 10:95-105.
Seung HS, Lee DD, Reis BY, Tank DW (2000) Stability of the memory of eye position in a recurrent network of conductance-based model neurons. Neuron. 26:259-271.
Shelton DP (1985) Membrane resistivity estimated for the Purkinje neuron by means of a passive computer model. Neuroscience 14:111-131.
Silver RA, Momiyama A, Cull-Candy SG (1998) Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-porkinje cell synapses. J. Physiol. (Lond.). 510:881-902.
Stuart G, Häusser M (1994) Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron. 13:703-712.
Turrigiano G, LeMasson G, Marder E (1995) Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J. Neurosci. 15:3640-3652.
Usowicz MM, Sugimori M, Cherksey B, Llinás R (1992a) Characterization of P-type calcium channels in cerebellar Purkinje cells. Neurosci. Abstr. 18:974.
Usowicz MM, Sugimori M, Cherksey B, Llinás R (1992b) P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 9:1185-1199.
Vanier MC, Bower JM (1999) A comparative survey of automated parameter-search methods for compartmental neural models. J. Comp. Neurosci. 7:149-171.
Wang X-J (1999) Fast burst firing and short-term synaptic plasticity: A model of neocortical chattering neurons. Neuroscience 89:347-362.
Wang Y, Strahlendorf JC, Strahlendorf HK (1991) A transient voltage-dependent outward potassium current in mammalian cerebellar Purkinje cells. Brain Res. 567:153-158.
Wilson MA, Bower JM (1998) The simulation of large-scale neural networks. In: Koch C, Segev I. Methods in Neuronal Modeling. MIT Press, Cambridge, MA. pp. 291-334.
Xing J, Andersen RA (2000) Models of the posterior parietal cortex which perform multimodal integration and represent space in several coordinate frames. J. Cogn. Neurosci. 12:601-614.
Yamada WM, Koch C, Adams PR (1998) Multiple channels and calcium dynamics. In: Koch C, Segev I. Methods in Neuronal Modeling (2nd ed). MIT Press, Cambridge, MA. pp. 137-170.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coop, A.D., Reeke, G.N. The Composite Neuron: A Realistic One-Compartment Purkinje Cell Model Suitable for Large-Scale Neuronal Network Simulations. J Comput Neurosci 10, 173–186 (2001). https://doi.org/10.1023/A:1011269014373
Issue Date:
DOI: https://doi.org/10.1023/A:1011269014373