Abstract
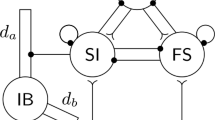
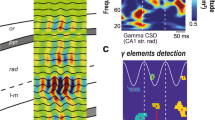
Gamma (30–80 Hz) and beta (12–30 Hz) oscillations such as those displayed by in vitro hippocampal (CA1) slice preparations and by in vivo neocortical EEGs often occur successively, with a spontaneous transition between them. In the gamma rhythm, pyramidal cells fire together with the interneurons, while in the beta rhythm, pyramidal cells fire on a subset of cycles of the interneurons. It is shown that gamma and beta rhythms have different properties with respect to creation of cell assemblies. In the presence of heterogeneous inputs to the pyramidal cells, the gamma rhythm creates an assembly of firing pyramidal cells from cells whose drive exceeds a threshold. During the gamma to beta transition, a slow outward potassium current is activated, and as a result the cell assembly vanishes. The slow currents make each of the pyramidal cells fire with a beta rhythm, but the field potential of the network still displays a gamma rhythm. Hebbian changes of connections among the pyramidal cells give rise to a beta rhythm, and the cell assemblies are recovered with a temporal separation between cells firing in different cycles. We present experimental evidence showing that such a separation can occur in hippocampal slices.
Similar content being viewed by others
References
Bibbig A (1999) Gamma-beta transition, pattern separation and the plasticity of inhibitory and excitatory synapses (abstract). Soc. Neurosci. Abs. 25: 2257.
Bibbig A (2000) Oscillations, synchronization, pattern separation and Hebbian plasticity in networks of excitatory and inhibitory cells (in German). Ph.D. Thesis, Department of Neural Information Processing and Department of Computational Science, University of Ulm, Germany.
Buhl EH, Tamas G, Fisahn A (1998) Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J. Physiol. 513(Pt. 1): 117–126.
Chawanya T, Aoyagi T, Nishikawa I, Okuda K, Kuramoto Y (1993) A model for feature linking via collective oscillations in the primary visual cortex. Biol. Cybern. 68: 483–490.
Chow C, White JA, Ritt J, Kopell N (1998) Frequency control in synchronized networks of inhibitory neurons. J. Comp. Neurosci. 5: 407–420.
Classen J, Gerloff C, Honda M, Hallett M (1998) Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J. Neurophysiol. 79(3): 1567–1573.
Crook SM, Ermentrout GB, Bower JM (1998) Spike frequency adaptation affects the synchronization properties of networks of cortical oscillations. Neural Comput. 10(4): 837–854.
Crook SM, Ermentrout GB, Vanier MC, Bower JM (1997) The role of axonal delay in the synchronization of networks of coupled cortical oscillators. J. Comp. Neurosci. 4: 11–172.
Doheny HC, Faulkner JH, Gruzelier T, Baldeweg T, Whittington MA (2000) Pathway specific habituation of induced gamma oscillations in the hippocampal slice. Neuroreport 11(12): 2629–2633.
Ermentrout GB, Kopell N (1998) Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc. Natl. Acad. Sci. 95: 1259–1264.
Farmer SF (1998) Rhythmicity, synchronization, and binding in human and primate motor systems. J. Physiol. 509(Pt. 1): 3–14.
Faulkner HJ, Traub RD, Whittington MA (1999) Anaesthetic/amnesic agents disrupt beta frequency oscillations associated with potentiation of excitatory synaptic potentials in the rat hippocampal slice. Br. J. Pharmacol. 128: 1813–1825.
Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291(5508): 1560–3156.
Fries P, Roelfsema PR, Engel AK, Konig P, Singer W (1997) Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc. Natl. Acad. Sci. 94(23): 12699–12704.
Gomez CM, Vazquez M, Vaquero E, Lopez-Mendoza D, Cardoso MJ (1998) Frequency analysis of the EEG during spatial selective attention. Int. J. Neurosci. 95(1-2): 17–32.
Grannan ER, Kleinfeld D, Somplinsky H (2000) Stimulus dependent synchronization of neuronal assemblies. Neural Comput. 5: 550–569.
Gray CM (1999) The temporal correlation hypothesis of visual feature integration: Still alive and well. Neuron 24: 31–47.
Gray CM, König P, Engel AK, Singer W (1989) Oscillatory responses in cat visual cortex exhibit intercolumnar synchronization which reflects global stimulus properties. Nature 338: 334–337.
Gross DW, Gotman J (1999) Correlation of high-frequency oscillations with the sleep-wake cycle and cognitive activity in humans. Neuroscience 94(4): 1005–1018.
Haenschel C, Baldeweg T, Croft R, Whittington MA, Gruzelier J (2000) Gamma and beta frequency cortical oscillation in response to novel auditory stimuli: A comparison of human electroencephalogram (EEG) data with in vitro models. Proc. Natl. Acad. Sci. 97(13): 7645–7650.
Halliwell JV (1982) M-current in human neocortical neurones. Neurosci. Lett. 67(1): 1–6.
Halliwell JV, Adams PR (1982) Voltageclamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 250: 71–92.
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117: 500–544.
Jefferys JGR, Traub RD, Whittington MA(1996) Neuronal networks for induced “40 Hz” rhythms. Trends Neurosci. 19: 202–207.
Jensen O, Lisman JE (1996a) Novel lists of 7+/-2 known items an be reliably stored in an oscillatory short-term memory network: Interaction with long-term memory. Learn Mem. 3(2-3): 257–263.
Jensen O, Lisman JE (1996b) Theta/gamma networks with slow NMDA channels learn sequences and encode episodic memory: Role of NMDA channels in recall. Learn Mem. 3(2-3): 264–278.
Joliot M, Ribary U, Llinas R (1994) Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc. Natl. Acad. Sci. 91: 11748–11751.
Jones SR, Pinto DJ, Kaper TJ, Kopell N (2000) Alpha-frequency rhythms desynchronize over long cortical distances: A modeling study. J. Comp. Neurosci. 9: 271–291.
Kienker P, Sejnowski J (1986) Separating figure from ground with a parallel network. Perception 15: 197–216.
König P, Schillen TB (1991) Stimulus-dependent assembly formation of oscillatory responses: I. Synchronization. Neural Comput. 3: 155–166.
Kopell N, Le Masson G (1994) Rhytmogenesis, amplitude modulation, and multiplexing in a cortical architecture properties. Proc. Natl. Acad. Sci. 91: 10586–10590.
Kopell N, Ermentrout GB, Whittington MA, Traub RD (2000) Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. 97: 1867–1872.
Lazzaro I, Gordon E, Whitmont S, Plahn M, Li W, Clarke S, Dosen A, Meares R (1998) Quantified EEG activity in adolescent attention deficit hyperactivity disorder. Clin. Electroencephalogr. 29(1): 37–42.
Li Z (1999) Visual segmentation by contextual influences via intracortical interactions in the primary visual cortex. Comput. Neural. Syst. 10: 187–212.
Liu X, Wang D (1999) Perceptual Organization Based on Temporal Dynamics. In: Moser MC, Jordan MI, Petsche T, eds. Advances in Neural Information Processing Systems 12. MIT Press, Cambridge, MA.
Müller MM, Bosch J, Elbert T, Kreiter A, Sosa MV, Sosa PV, Rockstroh B (1996) Visually induced gamma-band responses in human electroencephalographic activity: A link to animal studies. Exp. Brain. Res. 112(1): 96–102.
Pacheco P, Camperi M, Uchino T (2000) Parallel Neurosys: A system for the simulation of very large networks of biologically accurate neurons on parallel computers. Neurocomput. 32–33: 1095–1102.
Pfurtscheller G, Neuper C, Mohl W (1994) Event-related desynchronization (ERD) during visual processing. Intl. J. Psychophysiol. 16(2-3): 147–153.
Roelfsema PR, Engel AK, König P, Singer W (1997) Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 385: 157–161.
Schanze T, Eckhorn R (1997) Phase correlation among rhythms present at different frequencies: Spectral methods, application to microelectrode recordings from visual cortex and functional implications. Intl. J. Psychophysiol. 26(1-3): 171–189.
Shadlen M, Movshon JA (1999) Synchrony unbound:Acritical evaluation of the temporal binding hypothesis. Neuron 24: 67–77.
Sompolinski H, Golomb D (1991) Cooperative dynamics in visual processing. Phys. Rev. A 43: 6990–7011.
Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends Cog. Sci. 3(4): 151–162.
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998) Induced γ-band activity during the delay of a visual short-term memory task in humans. J. Neurosci. 18(1): 4244–4254.
Tallon-Baudry C, Kreiter A, Bertrand O (1999) Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans.Vis. Neurosci. 16(3): 449–459.
Terman D, Wang DL (1995) Global competition and local cooperation in a network of neural oscillators. Physica. D 81: 148–176.
Traub RD, Jefferys JGR, Whittington MA (1997) Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J. Comput. Neurosci. 4: 141–150.
Traub RD, Jefferys JGR, Whittington MA (1999a) Fast Oscillations in Cortical Circuits. MIT Press, Cambridge, MA.
Traub RD, Whittington MA, Buhl EH, Jefferys JGR, Faulkner H (1999b) On the mechanism of the gamma-beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J. Neurosci. 19(3): 1088–1105.
Traub RD, Whittington MA, Colling S, Buzsáki G, Jefferys JGR (1996a) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J. Physiol. 493: 471–484.
Traub RD, Whittington MA, Stanford IM, Jefferys JGR (1996b) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383: 621–625.
von Stein A, Rappelsberger P, Sarnthein J, Petsche H (1999) Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb. Cortex 9: 137–150.
Wang DL, Terman D (1997) Image segmentation based on oscillatory correlation. Neural Comput. 9: 1623–1626.
Wang X-J, Buzsáki G (1996) Gamma oscillation by synaptic inhibition in an interneuronal network model. J. Neurosci. 16: 6402–6413.
Whittington MA, Traub RD, Faulkner H, Stanford IM, Jefferys JGR (1997) Recurrent excitatory postsynaptic potentials induced by synchronized fast cortical iscillations. Proc. Natl. Acad. Sci. 94: 12198–12203.
Whittington MA, Traub RD, Jefferys JGR (1995) Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373(6515): 612–615.
Whittington MA, Traub RD, Kopell N, Ermentrout GB, Buhl EH (2000) Inhibition-based rhythms: Experimental and mathematical observations on network dynamic. Int. J. Psychophysiol. 38(3): 315–3336.
Yen S-C, Finkel L (1997) Salient Contour Extraction by Temporal Binding in a Cortically based Network. In: Moser MC, Jordan MI, Petsche T, eds. Advances in Neural Information Processing Systems 9. MIT Press, Cambridge, MA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Olufsen, M.S., Whittington, M.A., Camperi, M. et al. New Roles for the Gamma Rhythm: Population Tuning and Preprocessing for the Beta Rhythm. J Comput Neurosci 14, 33–54 (2003). https://doi.org/10.1023/A:1021124317706
Issue Date:
DOI: https://doi.org/10.1023/A:1021124317706