Abstract
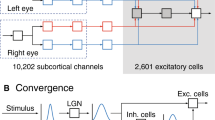
We present a model for development of orientation selectivity in layer IV simple cells. Receptive field (RF) development in the model, is determined by diffusive cooperation and resource limited competition guided axonal growth and retraction in geniculocortical pathway. The simulated cortical RFs resemble experimental RFs. The receptive field model is incorporated in a three-layer visual pathway model consisting of retina, LGN and cortex. We have studied the effect of activity dependent synaptic scaling on orientation tuning of cortical cells. The mean value of hwhh (half width at half the height of maximum response) in simulated cortical cells is 58° when we consider only the linear excitatory contribution from LGN. We observe a mean improvement of 22.8° in tuning response due to the non-linear spiking mechanisms that include effects of threshold voltage and synaptic scaling factor.
Similar content being viewed by others
References
Albus K, Wolf W (1984) Early postnatal development of neuronal functions in the kitten’s visual cortex: A laminar analysis. J. Physiol. 348: 153-185.
Alonso JM, Usrey WM, Reid RC (2001) Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J. Neurosci. 21: 4002-4015.
Arnett DW (1978) Statistical dependence between neighboring retinal ganglion cells in goldfish. Exp. Brain Res. 32: 49-53.
Arnett DW, Spraker TE (1981) Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J. Physiol. 317: 329-347.
Benevento LA, Creutzfeldt OD, Kuhunt U (1972) Significance of intracortical inhibition in the visual cortex. Nature 238: 124-126.
Blackmore C, Tobin EA (1972) Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp. Brain Res. 15: 439-440.
Blochl A, Thöenen H (1995) Characterization of nerve growth factor (NGF) release from hippocampal neurons. Evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur. J. Neurosci. 7: 1120-1228.
Bonds AB (1989) Role of inhibition in the specification of orientation selectivity of cells in the cat striate cortex. Vis. Neurosci. 2: 41-55.
Bonhoeffer T (1996) Neurotrophins and activity dependent development of the neocortex. Curr. Opin. Neurobiol. 6: 119-126.
Buzas P, Eysel UT, Adorjan P, Kisvarday ZF (2001) Axonal topography of cortical basket cells in relation to orientation, direction and ocular dominance maps. J. Comp. Neurol 437: 259-285.
Carandini M, Ferster D (2000) Membrane potential and firing rate in cat primary visual cortex. J. Neurosci. 20(1): 470-484.
Cellerino A, Maffei L (1996) The action of neurotrophins in the development and plasticity of the visual cortex. Progress in Neurobiol. 49: 53-71.
Chapman B, Zahs KR, Stryker MP (1991) Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J. Neurosci. 11: 1347-1358.
Cheng H, Chino Y, Smith E, Hamamoto J, Yoshida K (1995)Transfer characteristics of lateral geniculate nucleus X neurons in the cat: Effects of spatial frequency and contrast. J. Neurophysiol. 74: 2548-2557.
Chung S, Ferster D (1998) Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20: 1177-1189.
Crair MC, Gillespie DC, Stryker MP (1998) The role of visual experience in the development of columns in cat visual cortex. Science 279: 566-570.
Creutzfeldt OD, Kuhunt U, Benevento LA (1974) An intracellular analysis of visual cortical neurons to moving stimuli: Responses in a cooperative neuronal network. Exp. Brain Res. 21: 251-274.
Daugman JG (1985) Uncertainty relation for resolution in space, spatial frequency, and orientation optimized by two-dimensional visual cortical filters. J. Opt. Soc. America A 2: 1160-1169.
DeAngelis GC, Anzai A, Ohzawa I, Freeman RD (1992) Spatiotemporal receptive field structure and phase relationships between adjacent simple cells in the cats striate cortex. Soc. Neurosci. Abstr. 18: 10.
DeAngelis GC, Ohzawa I, Freeman RD (1993) Spatiotemporal organization of simple cell receptive fields in the cat’s striate cortex-I. General Characteristics and postnatal development. J. Neurophysiol. 69(4): 1091-1117.
DeAngelis GC, Ohzawa I, Freeman RD (1995) Receptive field dynamics in central visual pathways. TINS. 18: 451-458.
Douglas RJ, Martin KA, Whitteridge D (1991) An intracellular analysis of the visual responses of neurons in cat visual cortex. J. Physiol. 440: 659-696.
Elliott T, Howarth CI, Shadbolt NR (1996) Axonal processes and neural plasticity. I: Ocular dominance columns. Cerebral Cortex. 6: 781-788.
Elliott T, Shadbolt NR (1998) Competition for Neurotrophic factors: Ocular dominance Columns. J. Neurosci. 18(15): 5850-5858.
Elliott T, Shadbolt NR (2002) Multiplicative synaptic normalization and a nonlinear hebb rule underlie a neurotrophic model of competitive synaptic plasticity. Neural Computation. 14: 1311-1322.
Enroth-Cugell C, Robson JG (1966) The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 187: 517-552.
Erwin E, Miller KD (1998) Correlation based development of ocularly matched orientation and ocular dominance maps: Determination of required input activity structures. J. Neurosci. 18: 9870-9895.
Eysel UT, Crook JM, Machemer HF (1990) GABA-induced remote inactivation reveals cross-orientation inhibition in the cat striate cortex. Exp. Brain Res. 80: 626-630.
Ferster D (1986) Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J. Neurosci. 6(5): 1284-1301.
Ferster D, Chung S, Wheat H (1996) Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380: 249-252.
Gardner JL, Anzai A, Ohzawa I, Freeman RD (1999) Linear and nonlinear contributions to orientation tuning of simple cells in the cat’s striate cortex. Vis. Neurosci. 16: 1115-1121.
Gerstner W (1999) Spiking neurons. In: W Mass, CM Bishop, eds. Pulsed Neural Networks. MIT Press, Cambridge, pp. 3-54.
Goodhill GJ (1993) Topography and ocular dominance: A model exploring positive correlations. Biol. Cybern. 69: 109-118.
Goodhill GJ, Barrow HG (1994) The role of weight normalization in competitive learning. Neural Comp. 6: 109-118.
Griesbeck O, Paradanian AS, Sendrner M, Thoenen H (1995) Expression of neurotrophins in skeletal-muscle-quantitative comparison and significance for motoneuron survival and maintenance of function. J. Neurosci. Res. 42(1): 21-33.
Guillery RW (1988) Competition in the development of visual pathways. In: JG Parnavelas, CD Stern, RV Stirling, eds. The Making of Nervous System. Oxford University Press, Oxford. pp. 356-379.
Harris AE, Ermentrout GB, Small SL (1997) A model of ocular dominance column development by competition for trophic factor. Proc. Natl. Acad. Sci. USA 94: 9944-9949.
Hata Y, Tsumoto T, Sato H, Hagihara K, Tamura H (1988) Inhibition contributes to orientation selectivity in visual cortex of cat. Nature 335: 815-817.
Hayes WP, Meyer RL (1988) Optic synapse number but not density is constrained during regeneration onto surgically halved tectum in goldfish: HRP-EM evidence that optic fibers compete for fixed number of exuberant optic fibers is activity dependent. J. Neurosci. 9: 1414-1423.
Hochstein S, Shapley RM (1976) Quantitative analysis of retinal ganglion cell classifications. J. Physiol. 262: 237-264.
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 160: 106-154.
Jones JP, Palmer LA (1987) The two-dimensional spatial structure of simple receptive fields in cat striate cortex. J. Neurophysiol. 58: 1187-1211.
Kisvarday ZF, Toth E, Rausch M, Eysel UT (1997) Orientation specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of cat. Cerebral Cortex 7: 605-618.
Lampl I, Anderson JS, Gillespie DC, Ferster D (2001) Prediction of orientation selectivity from receptive field architecture in simple cells of the cat visual cortex. Neuron. 30: 263-274.
Lissen DV, Comperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, Von Zastrow M (1998) Activity differentially regulates the surface expression of synapticAMPAandNMDAglutamate receptors. Proc. Nat. Acad. Sci. 95: 7097-7102
Mastronarde DN (1983a) Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X and Y cells. J. Neurophysiol. 49: 303-324.
Mastronarde DN (1983b) Correlated firing of cat retinal ganglion cells. II. Responses of X and Y cells to single quantal events. J. Neurophysiol. 49: 335-349.
Miller KD (1990) Derivation of linear hebbian equations from a non-linear hebbian model of synaptic plasticity. Neural Comp. 2: 321-333.
Miller KD (1994) A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between ON and OFF center inputs. J. Neurosci. 14: 409-441.
Miller KD (1996) Synaptic economics: Competition and cooperation in a correlation based synaptic competition. Neuron. 17: 371-374.
Miller KD (1998) Equivalence of a sprouting-and-retraction model and correlation-based plasticity models of neural development. Neural Comp. 10: 529-547.
Miller KD, Keller JB, Stryker MP (1989) Ocular dominance column development: Analysis and simulation. Science 245: 605-615.
Miller KD, MacKay DJC (1994) The role of constraints in hebbian learning. Neural Comp. 6: 100-126.
Mooney R, Penn AA, Gallego R, Shatz CJ (1996) Thalamic relay of spontaneous retinal activity prior to vision. Neuron 17: 863-874.
Movhson JA, Thompson ID, Tolhurst DJ (1978) Spatial summation in receptive fields of simple cells in the cat striate cortex. J. Physiol. 283: 53-77.
Pei X, Vidyasagar TR, Volgushev M, Creutzfeldt OD (1994) Receptive field analysis and orientation selectivity of postsynaptic potentials of simple cells in cat visual cortex. J. Neurosci. 14(11): 7130-7140.
Piepenbrock C, Ritter H, Obermayer K (1997) The joint development of orientation and ocular dominance: Role of constraints. Neural Computation 9(5): 959-970.
Pockett S, Slack JR (1982) Pruning of axonal trees results in increased efficacy of surviving nerve terminals. Brain Res. 243: 350-353.
Purves D (1994) Neural activity and the growth of the brain. Cambridge University Press, Cambridge.
Purves D, Lichtman JW (1985) Principles of Neural Development. Sunderland, MA: Sinauer.
Reid RC, Alonso JM (1995) Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378: 281-264.
Roerig B, Chen B (2002) Relationship of local inhibitory and excitatory circuitry to orientation preference maps in ferret visual cortex. Cereb. Cortex. 12: 187-198.
Sabel BA, Schneider GE (1988) The principle of ‘conservation of total axonal arborization’ massive compensatory sprouting in the hamster subcortical visual system after early tectal lesions. Exp. Brain Res. 73: 505-518.
Schumann EM, Madison DV (1994) Locally distributed synaptic potentiation in the hippocampus. Science 263: 532-536.
Schummers JM, Mario J, Sur M (2001) Orientation tuning of intracellular potentials and spike responses at pinwheel centers and iso-orientation domains in primary visual cortex. Soc. Neurosci. Abst. 27: 619.19.
Sherk H, Stryker MP (1976) Quantitative study of orientation selectivity in visually inexperienced kittens. J. Neurophysiol. 39: 63-70.
Sillito AM, Kemp JA, Milson JA, Berardi N (1980) A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res. 194: 517-520.
Skottun BC, DeValois RL, Grosof DH, Movhson JA, Albrecht DG, Bonds AB (1991) Classifying simple and complex cells on the basis of response modulation. Vis. Res. 31: 1079-1086.
Somers DC, Nelson SB, Sur M (1995) An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15: 5448-5465.
Sur M (2002) Private communications.
Sur M, Leamey CA (2001) Development and plasticity of cortical areas and networks. Nature Reviews Neuroscience 2: 251-262.
Swindale NV (1996) The development of topography in the visual cortex:Areviewof models. Network: Computation in Neural Systems 7: 161-247.
Turrigiano GG (1999) Homeostatic plasticity in neuronal networks: The more things change, the more they stay the same. Trends Neurosci. 22: 221-227.
Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998) Activity dependent scaling of quantal amplitude in neocortical neurons. Nature 391(26): 892-896.
Van Ooyen A (2001) Competition in the development of nerve connections: A review of models. Network: Comput. in Neural Syst. 12: R1-R47.
Volgushev M, Pemberg J, Eysel UT (2000) Comparison of the selectivity of postsynaptic potentials and spike responses in cat visual cortex. Eur. J. Neurosci. 12: 257-263.
Von der Malsburg C (1973) Self organization of orientation selective cells in the striate cortex. Kybernetik 14: 85-100.
Weliky M, Kandler K, Fitzpatrick D, Katz LC (1995) Patterns of excitation and inhibition evoked by horizontal connections in visual cortex share a common relationship to orientation columns. Neuron 15: 541-552.
Weliky M, Katz LC (1999) Correlational structure of spontaneous neuronal activity in developing lateral geniculate nucleus in vivo. Science 285: 599-604.
Wiesel TN, Hubel DH (1974) Ordered arrangement of orientation columns in monkeys lacking visual experience. J. Comp. Neurol. 158: 307-318.
Willshaw DJ (1981) The establishment and the subsequent elimination of polyneural innervation of developing muscle: Theoretical considerations. Proc. R. Soc. B. 212: 233-252.
Wörgötter F, Koch C (1991) A detailed model of the primary visual pathway in the cat: Comparison of afferent excitatory and intracortical inhibitory connection schemes for orientation selectivity. J. Neurosci. 11(7): 1959-1979.
Xiong M, Pallas SL, Lim S, Finlay BL (1994) Regulation of retinal ganglion cell axon arbor size by target availability: Mechanism of compression and expansion of the retinotectal projection. J. Comp. Neurol. 344: 581-597.
Yousef T, Toth E, Rausch M, Eysel UT, Kisvarday ZF (2001) Topography of orientation center connections in the primary visual cortex of the cat. Neuro Report 12(8): 1693-1699.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhaumik, B., Mathur, M. A Cooperation and Competition Based Simple Cell Receptive Field Model and Study of Feed-Forward Linear and Nonlinear Contributions to Orientation Selectivity. J Comput Neurosci 14, 211–227 (2003). https://doi.org/10.1023/A:1021911019241
Issue Date:
DOI: https://doi.org/10.1023/A:1021911019241