Abstract
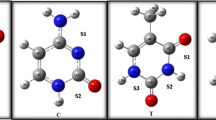
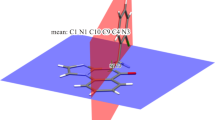
In this paper, ab initio calculated NQR parameters for some quinoline-containing derivatives are presented. The calculations are carried out in a search for the relationships between the charge distribution of these compounds and their ability to interact with haematin. On the basis of NQR parameters, π-electron density on the nitrogen atom of the quinoline ring plays a dominant role in determining the ability of quinolines to interact with haematin. This point was confirmed with investigation of Fe+3 cation-π quinoline ring interactions in 2- and 4-aminoquinoline. However, our results do not show any preference for those carbon atoms of the quinoline ring which previous reports have noted. In order to calculate the NQR parameters, the electric field gradient (EFG) should be evaluated at the site of a quadrupolar nucleus in each compound. EFGs are calculated by the Gaussian 98 program using the B3LYP/6-31G* level of theory.
Similar content being viewed by others
References
Graybeal, J.D., Molecular Spectroscopy. McGraw-Hill, New York, 1998.
Hadipour, N.L., Rafiee, M.A., Javaheri, M. and Mousavi, M.K., Chem. Phys. Lett., 356 (2002) 445.
World Health Organization, The World Health Report, 1999.
Warhurst, D.C., Biochem. Pharmacol., 30 (1981) 3323.
Sugioka, Y., Suzuki, M., Sugioka, K. and Nakano, M.A., FEBS Lett., 223 (1987) 251.
Blauer, G., Akkawi, M. and Bauminger, E.R., Biochem. Pharmacol., 46 (1993) 1573.
Adams, P.A., Berman, P.A.M., Egan, T.J., Marsh, P.J. and Silver, J.J., Inorg. Biochem., 63 (1996) 69.
Egan, T.J., Mini Rev. Med. Chem., 1 (2001) 113.
Vippagunta, S.R., Dorn, A., Matile, H., Bhattacharjee, A.K., Karle, J.M., Ellis, W.Y., Ridley, R.G. and Vennerstrom, J.L., J. Med. Chem., 42 (1999) 4630.
Egan, T.J., Hunter, R., Kaschula, C.H., Marques, H.M., Misplon, A. and Walden, J., J. Med. Chem., 43 (2000) 283.
Egan, T.J., Mavuso, W.W., Ross, D.C. and Marques, H.M., J. Inorg. Biochem., 68 (1997) 137.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A., Stratmann, Jr., R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G. A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S. and Pople, J.A., Gaussian 98. Gaussian, Inc., Pittsburgh, PA, 1998.
Kawakami, J., Miyamoto, R., Kimura, K., Obata, K., Nagaki, M. and Kitahara, H., J. Comput. Chem. Jpn., 2 (2003) 57.
Coussan, S., Manca, C., Tanner, C., Bach, A. and Leutwyler, S., J. Chem. Phys., 119 (2003) 3774.
Slanina, Z., Hsu, M. and Chow, T.J., J. Chin. Chem. Soc., 50 (2003) 593.
Becke, A.D., J. Chem. Phys., 98 (1993) 5648.
Lee, C., Yang, W. and Parr, R.G., Phys. Rev. B, 37 (1988) 785.
Miehlich, B., Savin, A., Stoll, H. and Preuss, H., Chem. Phys. Lett., 157 (1989) 200.
Lucken, E.A.C., Nuclear Quadrupole Coupling Constant. Academic Press, London, 1969.
Cohen, M.H. and Reif, F., Solid State Phys., 5 (1975) 321.
Hadipour, N.L., Rafiee, M.A. and Javaheri, M., Chem. Phys. Lett., 366 (2002) 578.
Pyykko, P., Mol. Phys., 99 (2001) 1617.
Chou, A.C., Chevli, R. and Fitch, C.D., Biochemistry, 19 (1980) 1543.
Egan, T.J., Mavuso, W.W., Ross, D.C. and Marques, H.M., J. Inorg. Biochem., 68 (1997) 137.
Sullivan, D.J. Jr., Gluzman, I.Y., Russell, D.G. and Goldberg, D.E., Proc. Natl. Acad. Sci. USA, 93 (1996) 11865.
Hunter, C.A. and Sanders, J.K.M., J. Am. Chem. Soc., 112 (1990) 5525.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rafiee, M.A., Hadipour, N.L. & Naderi-manesh, H. A correlation study of quinoline derivatives and their pharmaceutical behavior by ab initio calculated NQR parameters. J Comput Aided Mol Des 18, 215–220 (2004). https://doi.org/10.1023/B:JCAM.0000035201.67977.16
Issue Date:
DOI: https://doi.org/10.1023/B:JCAM.0000035201.67977.16