Paper:
Study on Pipetting Motion Optimization of Automatic Spheroid Culture System for Spheroid Formation
Takeshi Shimoto*1, Chihiro Teshima*1, Toshiki Watanabe*1, Xiu-Ying Zhang*2, Atsushi Ishikawa*3, Hidehiko Higaki*3, and Koichi Nakayama*4
*1Fukuoka Institute of Technology
3-30-1 Wajiro-higashi, Higashi-ku, Fukuoka 811-0295, Japan
*2Kyushu University
3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan
*3Kyushu Sangyo University
2-3-1 Matsukadai, Higashi-ku, Fukuoka 813-8503, Japan
*4Saga University
5-1-1 Nabeshima, Saga 849-8501, Japan
This research group has established a technology for producing a three-dimensional cell constructed using only the cell itself. This technology uses a property in which the spheroids fuse with each other. We developed a system that automates the spheroid production process to obtain reproducible spheroids and suppress variation factors that occur from human operation. However, it has become clear that the dispersion occurs in the diameter depending on the number of cells of the spheroid even if the cells are handled in the same manner. The purpose of this research is to examine an appropriate pipetting motion in accordance with the number of cells of the spheroid to be produced. Rabbit mesenchymal stem cells (rMSCs) are used as the objects. The number of cells was set to 2×104, 3×104, and 4×104 cells/well, and the passage number as 7. The appearance of spheroids cultured using the motion programmed in accordance with each number of cells was observed every 24 hours for 5 days after seeding. The results of the analysis indicate that the optimum motion in each number of cells has been successfully specified, and reproducible spheroids have been successfully produced.
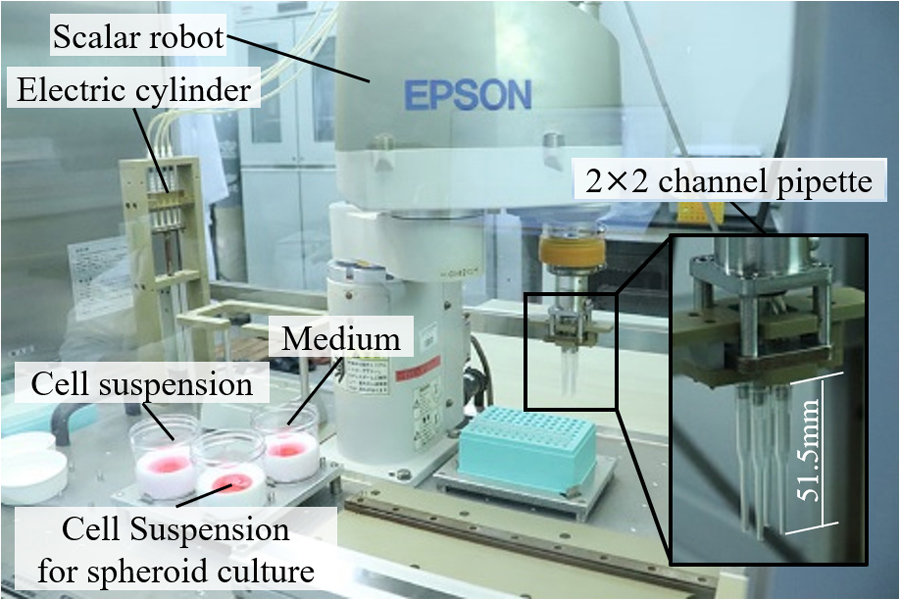
Automatic spheroid culture system
- [1] B. Gao, K. Sakaguchi, K. Matsuura, T. Ogawa, Y. Kagawa, H. Kubo, and T. Shimizu, “In Vitro production of human ballooned hepatocytes in a cell sheet-based three-dimensional model,” Tissue Engineering, Part A, Vol.26, No.1-2, pp. 93-101, 2020.
- [2] Y. Kasai, R. Takagi, S. Kobayashi, T. Owaki, N. Yamaguchi, H. Fukuda, Y. Sakai, Y. Sumita, N. Kanai, H. Isomoto, K. Kanetaka, T. Ohki, I. Asahina, K. Nagai, K. Nakao, N. Takeda, T. Okano, S. Eguchi, and M. Yamato, “A stable protocol for the fabrication of transplantable human oral mucosal epithelial cell sheets for clinical application,” Regenerative Therapy, Vol.14, pp. 87-94, 2020.
- [3] K. Arai, Y. Tsukamoto, H. Yoshida, H. Sanae, T. A. Mir, S. Sakai, T. Yoshida, M. Okabe, M. Nikaido, M. Taya, and M. Nakamura, “The development of cell-adhesive hydrogel for 3D printing,” Int. J. Bioprinting, Vol.2, No.2, pp. 44-53, 2016.
- [4] V. L. Tsang, A. A. Chen, L. M. Cho, K. D. Jadin, R. L. Sah, S. DeLong, J. L. West, and S. N. Bhatia, “Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels,” The FASEB J., Vol.21, pp. 790-801, 2006.
- [5] N. Minghao and T. Shoji, “Microfluidics based synthesis of coiled hydrogel microfibers with flexible shape and dimension control,” Sensors and Actuators B, Vol.246, pp. 358-362, 2017.
- [6] H. Onoe, T. Okitsu, A. Itou, M. Kato-Negishi, R. Gojo, D. Kiriya, K. Sato, S. Miura, S. Iwanaga, K. Kuribayashi-Shigetomi, Y. T. Matsunaga, Y. Shimoyama, and S. Takeuchi, “Metre-long cell-laden microfibres exhibit tissue morphologies and functions,” Nature Materials, Vol.12, No.6, pp. 584-590, 2013.
- [7] K. Kuribayashi-Shigetomi, H. Onoe, and S. Takeuchi, “Cell origami: self-folding of three-dimensional cell-laden microstructures driven by cell traction force,” PLOS ONE, Vol.7, No.12, e51085, 2012.
- [8] T. Shimoto, K. Nakayama, S. Matsuda, and Y. Iwamoto, “Building of HD MACs using cell processing robot for cartilage regeneration,” J. Robot. Mechatron., Vol.24, No.2, pp. 347-353, 2012.
- [9] N. Moldovan, N. Hibino, and K. Nakayama, “Principles of the kenzan method for robotic cell spheroid-Based three-dimensional bioprinting,” Tissue Engineering Part B, Vol.23, No.3, pp. 237-244, 2017.
- [10] K. Arai, D. Murata, A. R. Verissimo, Y. Mukae, M. Itoh, A. Nakamura, S. Morita, and K. Nakayama, “Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer,” PLOS ONE, Vol.13, No.12, e0209162, 2018.
- [11] X. Y. Zhang, Y. Yanagi, Z. Sheng, K. Nagata, K. Nakayama, and T. Taguchi, “Regeneration of diaphragm with bio-3D cellular patch,” Biomaterials, Vol.167, pp. 1-14, 2018.
- [12] D. Murata, K. Arai, and K. Nakayama, “Scaffold-Free Bio-3D Printing Using Spheroids as ”Bio-Inks” for Tissue (Re-) Construction and Drug Response Tests,” Advanced Healthcare Materials, Vol.9, Issue 15, 1901831, 2020.
- [13] K. Matsuura, “The maintenance of culture environment in 3D suspension culture,” The Japanese J. of Medical Instrumentation, Vol.85, No.4, pp. 419-423, 2015 (in Japanese).
- [14] T. Shimoto, A. Ishikawa, and H. Higaki, “Automation of spheroid formation process using cell processing robot for regenerative medicine,” Japanese J. of Clinical Biomechanics, Vol.39, pp. 305-311, 2018 (in Japanese).
- [15] S. Konagaya, T. Ando, T. Yamauchi, H. Suemori, and H. Iwata, “Long-term maintenance of human induced pluripotent stem cells by automated cell culture system,” Scientific Reports, Vol.5, 16647, 2015.
- [16] K. Matsuura, M. Wada, K. Konishi, M. Sato, U. Iwamoto, Y. Sato, A. Tachibana, T. Kikuchi, T. Iwamiya, T. Shimizu, J. K. Yamashita, M. Yamato, N. Hagiwara, and T. Okano, “Fabrication of mouse embryonic stem cell-derived layered cardiac cell sheets using a bioreactor culture system,” PLOS ONE, Vol.7, No.12, e52176, 2012.
- [17] L. Yuge, T. Kajiume, H. Tahara, Y. Kawahara, C. Umeda, R. Yoshimoto, S. L. Wu, K. Yamaoka, M. Asashima, K. Kataoka, and T. Ide, “Microgravity Potentiates Stem Cell Proliferation While Sustaining the Capability of Differentiation,” Stem Cells and Development, Vol.15, Issue 6, pp. 921-929, 2006.
- [18] T. Shimoto, X. Y. Zhang, A. Shizuka, I. Atsushi, H. Higaki, and K. Nakayama, “Analysis of cell spheroid morphological characteristics using the spheroid morphology evaluation system,” J. Robot. Mechatron., Vol.30, No.5, pp. 819-826, 2018.
- [19] Y. Yanagi, K. Nakayama, T. Taguchi, S. Enosawa, T. Tamura, K. Yoshimaru, T. Matsuura, M. Hayashida, K. Kohashi, Y. Oda, T. Yamaza, and E. Kobayashi, “In vivo and ex vivo methods of growing a liver bud through tissue connection,” Scientific Reports, Vol.7, 14085, 2017.
- [20] Y. Sakai, S. Yoshida, Y. Yoshiura, R. Mori, T. Tamura, K. Yahiro, H. Mori, Y. Kanemura, M. Yamasaki, and K. Nakazawa, “Effect of microwell chip structure on cell microsphereproduction of various animal cells,” J. of Bioscience and Bioengineering, Vol.110, No.2, pp. 223-229, 2010.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.