Abstract
This paper reports a study of the impact of differences in meal ingestion amount on electrogastrograms. The study was performed by recording an electrogastrogram and an electrocardiogram of eight young men for 60 min once before and once after each test subject ingested meals with ingestion amounts of 800 kcal and 400 kcal. The results showed that meal ingestion affected the power spectral density of the tachygastria range (3.7–5.0 cpm) by significantly increasing its value after the meal. The differences in meal ingestion amount are expressed in the power spectral density of the colon range (6.0–8.0 cpm) by significantly increasing its value after the meal, but only when the subject ingested an 800-kcal meal.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The aging of the global population is accompanied by a steady increase in expectations regarding the health care field. The rate of aging in Japan is among the highest in the world, attracting companies’ interest in medical treatment and health care from various foreign countries. A problem facing aging societies is a rise in the number of elderly people requiring nursing care, accompanied by the rising cost of medical treatment and burden of nursing care. Thus, the problem of how to reduce the number of elderly people who require nursing care is one challenge that must be overcome quickly. In addition, abnormal peristaltic movement of the gastrointestinal tract is related to a decline in physical and psychological quality of life, so early discovery and prompt treatment of abnormalities in peristaltic movement is an effective way to overcome this challenge [1].
One type of alimentary canal obstruction movement function investigation is an electrogastrogram (EGG), which is a noninvasive low-constraint measurement of the electrical activity of the gastrointestinal tract performed from the body surface [2, 3]. Regular electrical activities that repeatedly electrically depolarize and repolarize are seen in the stomach and intestines, just as in the heart. Pacemaker cells that control the electrical activity of the stomach are in the top 1/3 of the greater curvature of the stomach, and from there, electrical activity in the body is transmitted toward the pyloric region at a rate of three waves per minute (three cycles per minute (cpm)). The pacemaker cells are governed by parasympathetic nervous system activities but cause spontaneous cyclical electrical activities. This is a result of a network of islands called interstitial cells of Cajal (ICCS) [4,5,6,7]. This electrical activity is categorized as either electrical response activity (ERA), which is accompanied by peristaltic movement, or electrical control activity (ECA), which is not accompanied by peristaltic movement [8], but an electrogastrogram cannot distinguish between these, so peristaltic movement is not directly recorded [9]. However, an electrogastrogram definitely records the electrical activity of the stomach [10], and it is thought to be possible to discover abnormal peristaltic movement based on a response confirmation test.
In the case where it is difficult to visually assess the waveform shown on an electrogastrogram, it is necessary to perform spectral analysis of the waveforms shown on the electrogastrogram. The dominant frequency of the electrogastrogram obtained by spectral analysis is considered to be 2.4–3.7 cpm, with those smaller than 2.4 cpm considered to be bradygastria, and those larger than 3.7 cpm considered to be tachygastria.
Response confirmation of an electrogastrogram during meal ingestion often uses enteral nutrition or solid nutritional supplements, etc., and from 5 to 15 min after meal ingestion by a healthy person, its period falls to between 0.2 and 0.5 and its amplitude increases by 1 to 3 times. This response is called “postprandial dip” [11, 12]. Regarding postprandial dip, there is no reference to its impact in frequency ranges outside of the dominant frequency, and the specific ingestion amount that causes postprandial dip is not clear. This research studied the impact of differences in meal ingestion amount on electrogastrograms. A study comparing a measured electrogastrogram with the numerical solution obtained from a stochastic differential equation the authors propose was also performed to determine the impact of differences in meal ingestion amount on the mathematical model of an electrogastrogram.
2 Experimental Method
2.1 Subjects and Materials
The subjects were eight young men between 21 and 28 years of age (average ± standard differential: 22.8 ± 1.4) who had no previous history nor presented symptoms of digestive disease. The experiment was first fully explained to the subjects, and their agreement to participate in the experiment was obtained by having them enter answers on a form that presented the purpose and significance of the research, guarantee of privacy, handling of data. The electronic data obtained in the experiment were recorded using aliases that cannot be linked to the test subjects, and the experiment obtained the approval of the Ethics Committee of Toyama Prefectural University.
2.2 Protocol
The experiments were performed by recording electrogastrograms and electrocardiograms for 60 min with the subjects resting in a supine position before and after meal ingestion. The meal load used solid portable foods, and the test subjects ingested 800-kcal meals and 400-kcal meals. The measurements of each test subject were performed on different days, and considering the effects of sequence, the sequences were set randomly.
The electrogastrograms were acquired using disposable electrodes used to perform electrocardiograms (blue sensors, METs), applied as shown in Fig. 1. The electrodes were applied after skin resistance was adequately lowered using disinfection-use ethanol. Each electrogastrogram was recorded using bipolar leads, amplification was performed by a biological amplifier (Biotop mini, East Medic Corporation), and the data were recorded on a PC using an analog input/output card (ADA16-32/2(CB)F, CONTEC). The biological amplifier measurement conditions were a low pass filter of 0.02 Hz and a high pass filter of 0.5 Hz. To unify the time food was retained in the stomachs of all the subjects, they were ordered not to eat for two hours prior to the start of the experiment.
2.3 Analytical Indices
In this study, time series data were obtained by A/D conversion of the electrogastrograms and electrocardiograms, which were recorded at 1 kHz. In the electrogastrogram time series, to remove electrical noise caused by the foreign electromyogram or electronic equipment, a band pass filter with a cutoff frequency from 0.015–0.15 Hz was applied to the time series data that were obtained. In addition, because the normal frequency of an electrogastrogram has a relatively slow fluctuation of approximately 3 cpm, the 1-kHz electrogastrogram time series was resampled at 1 kHz. This research applied running spectral analysis to analyze the time series that was obtained. The electrogastrogram time series was shifted and divided between 1,024 points (approx. 17 min) time windows at 300 point intervals, and each was analyzed. In this paper, among analysis intervals, divided start times are entered as the representative value of each analysis interval, as shown below.
The divided electrogastrogram time series used a translational error estimated statistically according to a Wayland algorithm in addition to frequency analysis [13, 14]. The translational error (Etrans) estimated according to the Wayland algorithm is an index that quantitatively evaluates the smoothness of the track of an attractor embedded in topological space. If the track of an attractor reconstituted in embedded space is smooth, the time series has determinism. If the translational error is a positive value and is close to zero, and the numerical model that forms the time series is deterministically large, it can be considered probabilistic. When the object has Brownian motion, in particular, the value of the translational error is estimated to be 1.
The frequency analysis of each electrogastrogram time series was accomplished by performing the maximum entropy method (MEM) for each of the divided time histories and employing frequency analysis using a stochastic method [15, 16]. As the spectrum calculation method for use in the MEM, algorithms based on the Yule-Walker method and Burg method have been proposed, but for this study, the Burg method was adopted because it permits stable spectrum estimation with high resolution, even using sparse data. In this paper, the smallest value for which the autocorrelation function is 1/e or lower (correlation is considered to be zero) was defined as the lag value of the autocorrelation function. From the power spectrum that was calculated, the power spectral density (PSD) in these frequency bands was calculated focusing on bradygastria (1.1–2.4 cpm) and tachygastria (3.7–5.0 cpm) [17]. It has been reported that fluctuation of approximately 7 cpm in an electrogastrogram reflects the electrical activity of the colon [18], so PSD was also calculated for the 6.0–8.0 cpm range.
The electrocardiograms recorded at the same time as the electrogastrograms were analyzed based on heart rate variability (HRV). HRV can quantify indices of the sympathetic nervous system and the parasympathetic nervous system by analyzing the RR interval, which is the stroke of the heart from the time range and frequency range. The RR interval time series that was abstracted was divided by shifting time windows at 512 points at 300-s intervals, and each was analyzed, corresponding to the divided times of the electrogastrogram time series. In this paper, with the low-frequency component LF of PSD as 0.04–0.15 Hz, and the high-frequency component HF of PSD as 0.15–0.4 Hz, HF, which represents the activity index of the parasympathetic nerves, and LF/HF, which represent the activity index of the sympathetic nerves, were separately calculated.
For the calculated analysis indices, the average values recorded for each time before meal ingestion and for each time after meal ingestion were compared using the Wilcoxon signed-rank test (this study used a significance level of .05).
3 Results
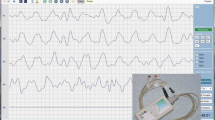
Figure 2 shows typical examples of electrogastrogram waveforms from 10 min to 20 min after start of measurement for the same test subject. Regardless of the meal ingestion amount, the measured waveform after meal ingestion has a higher amplitude than that before meal ingestion. In addition, the measured waveform when 400 kcal was ingested was compared with the measured waveform when 800 kcal was ingested, showing a clear change of 3 cpm. In the measured waveform when 800 kcal was ingested, on the contrary, the high-frequency component is irregularly superimposed by a change of approximately 3 cpm.
PSD and translational error in each range—bradygastria (1.1–2.4 cpm), tachygastria (3.7–5.0 cpm), and colon (6.0–8.0 cpm)—were calculated for divided electrogastrogram time series. The results for 800-kcal ingestion are shown in Fig. 3, and the results for 400-kcal ingestion are shown in Fig. 4. In the bradygastria range, consistent trends before and after meal ingestion, regardless of meal ingestion amount, were not seen (Figs. 3A and 4a), but in the tachygastria range, the value after meal ingestion was significantly higher than it was before meal ingestion (Figs. 3b, and 4b). This trend appeared beginning 5 min after meal ingestion and was sustained until 35 min after ingestion. In the colon range, when 400 kcal was ingested, no significant change was seen after meal ingestion; when 800 kcal was ingested, the value after meal ingestion was significantly higher than it was before meal ingestion (Figs. 3c and 4c). In the results for translational error, on the contrary, no consistent trend, regardless of the meal ingestion amount, was seen before or after meal ingestion (Figs. 3d and 4d).
Next, the electrocardiograms obtained at the same time as the electrogastrograms were analyzed. When a 400-kcal meal was ingested, both HF and LF/HF showed no significant difference before or after meal ingestion (Figs. 5b and 5d). When an 800-kcal meal was ingested, on the contrary, the HF was significantly reduced, and LF/HF tended to increase significantly. While HF showed this trend at all times after meal ingestion (Fig. 5a), LF/HF increased significantly from 0–20 min. Beginning at 25 min, it disappeared (Fig. 5c).
4 Discussion
This paper reports an experimental study of the impact of differences in meal ingestion amount on electrogastrograms. Response confirmation of an electrogastrogram when ingesting a meal showed that, in the tachygastria range (3.7–5.0 cpm) of an electrogastrogram, when ingesting meals of both 800 kcal and 400 kcal, the value of PSD was significantly higher after meal ingestion than before meal ingestion (p < 0.05). Moreover, because this tendency appeared more when an 800-kcal meal was ingested than when a 400-kcal meal was ingested, it is assumed that the meal ingestion affects the tachygastria range of an electrogastrogram. In the cases of ingestion of 800 kcal, in the colon range (6.0–8.0 cpm), the value of PSD was significantly higher after meal ingestion than before meal ingestion (p < 0.05). It is known that the movement of contents into the stomach or intestines caused by a meal encourages colon motility, causing peristaltic movement so strong that it cleans the contents out of the large intestine, a process called “mass movement.” This is called “gastrocolic reflex” and “small intestine gastrocolic reflex,” and is a mutual adjustment of the alimentary canal through extrinsic nerves [17]. These trends, which this experimental study has confirmed, can also be seen as improvement of intestinal motility through meal ingestion.
Analysis of the electrocardiograms recorded at the same time as the electrogastrograms confirmed that after ingestion of 800-kcal meals, HF declines significantly and LF/HF increases significantly. When ingesting a meal, the flow of blood into the abdomen increases and alimentary canal hormones expand blood vessels to improve gastrointestinal tract activity. The tendency shown by heart rate fluctuation analysis in 800-kcal ingestion cases can be seen in the restriction of improvement of sympathetic nerve activity and pneumogastric nerve activity by the actions that maintain apparent blood pressure as a result of the decline of peripheral vascular resistance throughout the body caused by the expansion of blood vessels [19, 20]. This is assumed to ensure improvement of activity in the colon range on the electrogastrograms after meal ingestion.
5 Conclusion
This paper reports a study of the impact of differences in meal ingestion amount on electrogastrograms. The results show that the meal ingestion affects the power spectral density in the tachygastria range of an electrogastrogram, significantly increasing its value after meal ingestion above its value before meal ingestion. Differences in meal ingestion amount also affect the power spectral density in the colon range, increasing its value significantly after meal ingestion above its level before meal ingestion only when an 800-kcal meal is ingested. These trends, which were confirmed by the electrogastrograms, can be seen as an improvement in intestinal activity by meal ingestion. In the future, we will aim to construct evidence that will be of use in early discovery of abnormal peristaltic movement of the gastrointestinal tract based on a multifaceted discussion of the complexity in the generators of an electrogastrogram using analysis by a mathematical model.
References
Aro, P., Talley, N.J., Agréus, L., et al.: Functional dyspepsia impairs quality of life in the adult population. Aliment. Pharmacol. Ther. 33(11), 1215–1224 (2011)
Alvarez, W.C.: The electrogastrogram and what is shows. J. Am. Med. Assoc. 78, 1116–1119 (1922)
Kenneth, L.K., Robert, M.: Handbook of Electrogastrography. Oxford University Press, Oxford (2004)
Nakamura, E., et al.: Cellular mechanism of spontaneous activity of the stomach smooth muscle. Nihon Yaku-rigaku Zasshi 123(3), 141–148 (2002)
Torihashi, S.: Structure and functions of the Cajal cells. Pediatr. Surg. 37(4), 467–472 (2005)
Takayama, I., Horiguchi, K., Daigo, Y., Mine, T., Fujino, M.A., Ohno, S.: The interstitial cells of Cajal and a gastroenteric pacemaker system. Arch. Histol. Cytol. 65(1), 1–26 (2002)
Thomsen, L., et al.: Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 4, 848–851 (1998)
Smout, A.J.P.M., Van Der Schee, E.J., Grashuis, J.L.: What is measured in Electrogas-trography? Dig. Dis. Sci. 25(3), 179–187 (1980)
Chen, J.Z., McCallum, R.W.: Electrogastrography: Principles and Applications. Raven Press, Ely (1994)
Pezzolla, F., Riezzo, G., Maselli, M.A.: Electrical activity recorded from abdominal surface after gastrectomy or colectomy in humans. Gastroenterology 97(2), 313–320 (1989)
Sakakibara, Y., Asahina, M., Suzuki, A., et al.: Gastric myoelectrical differences between Parkinson’s disease and multiple system atrophy. Mov. Disord. 24, 1579–1586 (2009)
Seligman, W.H., Low, D.A., Asahina, M., Mathias, C.J.: Abnormal gastric myoelectrical activity in postural tachycardia syndrome. Clin. Auton. Res. 23(2), 73–80 (2013)
Wayland, R., Bromley, D., Pickett, D., Passamante, A.: Recognizing determinism in a time series. Phys. Rev. Lett. 70(5), 580–582 (1993)
Takada, H., Simizu, Y., Hoshita, H., Shiozawa, T.: Wayland tests for differenced time series could evaluate degrees of visible determinism. Bull. Soc. Sci. 19(3), 301–310 (2005)
Hino, M.: Spectral Analysis. Asakura Shoten, Tokyo (1977)
Minami, S.: Handling Wave Form Data for Scientific Measurements. CQ Shuppansha, Tokyo (1986)
Japan Society of Neurovegetative Research: Autonomic nerve function examination. vol. 5. Bunkodo Co., Ltd. (2007)
Homma, S.: Isopower mapping of the electrogastrogram (EGG). J. Auton. Nerv. Sys. 62(3), 163–166 (1997)
Koike, Y.: Differences between postprandial hypotension and orthostatic hypotension. Did you know? Postprandial hypotension – New blood pressure abnormality clinic, Nanzando, pp. 80–83 (2004)
Hirayama, M.: Expression mechanisms from the perspective of hemodynamics. Did you know? Postprandial hypotension – New blood pressure abnormality clinic, Nanzando, pp. 93–99 (2004)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Kinoshita, F., Miyanaga, K., Fujita, K., Touyama, H. (2019). Effect of Differences in the Meal Ingestion Amount on the Electrogastrogram Using Non-linear Analysis. In: Antona, M., Stephanidis, C. (eds) Universal Access in Human-Computer Interaction. Multimodality and Assistive Environments. HCII 2019. Lecture Notes in Computer Science(), vol 11573. Springer, Cham. https://doi.org/10.1007/978-3-030-23563-5_37
Download citation
DOI: https://doi.org/10.1007/978-3-030-23563-5_37
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23562-8
Online ISBN: 978-3-030-23563-5
eBook Packages: Computer ScienceComputer Science (R0)