Abstract
Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI) are cognitive diminished conditions that requires neuropsychological, images and complementary test for diagnosis. The electroencephalogram equipment is a less expensive, less invasive and a more portable option that image ones so there is an increased interest in an EEG based methodology for cognitive impairment diagnosis. In this work is presented an eyes closed resting state condition EEG signal diagnosis tool, based on wavelet decomposition and wavelet entropy. The methodology allows discriminating among Healthy, Mild Cognitive Impairment and Alzheimer’s disease people. For this purpose theta band-EEG power ratio, beta band-EEG power ratio and entropy values distribution through time in 14 electrodes are used. Wavelet decomposition is performed on five levels using Haar wavelet mother on two seconds windows. After decomposition wavelet power ratio and entropy distribution calculation are performed. The characteristics are used in a Healthy–MCI, Healthy-AD and MCI-AD classification using Support Vector Machine with polynomial kernel providing six inputs to a neural network (two layer, 13 neurons in the hidden layer) in charge of the final classification. Data base is composed of 17 healthy, nine Mild Cognitive Impairment and 15 Alzheimer’s disease people registers. A precision of 92.68% to 97.56% is achieved, better or equal to other entropy-based methods with the advantage of separating the three groups and use a bigger database. This methodology reveals as a potential quantitative diagnosis-support tool especially between Healthy people and Mild Cognitive Impairment where some of the conventional test fails.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
German physician Alois Alzheimer first described the Alzheimer’s disease. It is a neurodegenerative disorder caused by senile plaques and neurofibrillary tangles, which provokes loss of neuron connectivity, brain inflammation, oxidative stress and cell death [1, 2]. Symptoms involve loss of recent memory, disorientation, poor judgment, apraxia and aphasia. Is preceded by a condition known as Mild Cognitive Impairment in which cognitive deterioration is not as large as in dementia but greater that the expected for the age. Diagnosis is based on family history, family description of patient behavior, cognitive, blood and images test for discarding other types of dementia or dementia-like syndromes. At this point, based on the Diagnostic and Statistical Manual of Mental Disorders version five (DSM 5) a MCI, AD or other type of dementia can be diagnosed [1,2,3].
The EEG signals is a register of the electrical activity in the brain through time. Since AD causes loss of connectivity and neurons death, it is expected that the EEG signal get affected. Known alterations are frequency diminution [4,5,6] (power increase in theta and delta band as well as a power decrease in alpha and beta) and complexity reduction in form of a loss of randomness [4, 6].
Entropy-based classification methodologies has been implemented using Tsallis, Multiscale, Sample, Permutation and Fuzzy entropies [7,8,9,10,11,12,13]. The best reported sensibilities and specificities lies between 72.73% to 90.91% [8] and 81.82% [13]. However only the MCI groups with healthy people, or AD with healthy people are studied.
In [14] a specificity of 100.0% and sensitivity of 98.0% is reached separating a healthy group and a MCI+AD group (named AD) with a single electrode (Fp1). The methodology is based on the absolute and relative energy of theta rhythm in eyes open condition (characteristic with the best performance), and wavelet entropy of the alpha rhythm in eyes closed (second best performance characteristic). Despite the results and the fact that Fp1 guarantees the recording of theta rhythm in the frontal region, the alpha rhythm is predominant in the occipital area. This leads to the fact that a displacement of the alpha wave towards frontal regions can be better detected with the use of electrodes in the frontal and occipital regions than with a single one. The number of people with MCI in the cognitive disorder group is not specified as being completely defined as Alzheimer’s. The total number of registers is 24. It is also considered that both groups are not completely paired since in the group of AD there are no individuals between 50 and 60 years old (3 in the control group) and few between 60 and 70 years old (1 in AD against four in the control group).
In this work, the main interest is to provide a methodology that could discriminate among the three possible clinical cases: Healthy people, MCI and AD. For this purpose wavelet entropy, theta band-EEG and beta band-EEG power ratio are used as classification characteristics. Its importance lies on the possibility of develop an auxiliary tool for specialists. This tool may help them to eliminate the uncertainty in the diagnosis, especially in MCI where some of the psychological tests provides specificity values of 17.1% to 65.7% and introduces false positives due to the subtle differences between normal ageing and MCI [15,16,17].
2 Materials and Methods
2.1 Subjects and EEG Recording
The EEG database is composed of 17 registers of healthy people (55 to 75 years old, mean 63.03 and standard deviation of 5.75), nine MCI registers (57 to 78 years old, mean 67.20 and standard deviation of 7.37) and 15 AD registers (57 to 85 years old, mean: 70.00 and standard deviation of 6.51). They were collected in Neurophysiology department at Juan Bruno Sayas General Hospital, Santiago de Cuba. A group of neuropsychologists using as exclusion criteria: psychiatric disorder, epilepsy, severe head trauma, stroke or ischemia performed the classification in Healthy, MCI and AD. The study was approved by the hospital Ethics Committee.
The EEG was acquired with subjects in supine position, resting state, alternating eyes closed and eyes open for eight minutes. It was used a 19 electrode, 10–20 international system referential montage (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz and Pz). The equipment consisted on a MEDICID 5 with 50 Hz/60 Hz programmable notch filter, sampling frequency of 200 Hz, analog to digital converter of 16 bits and band-pass of 0.5 Hz to 30 Hz. Signals were analyzed by a specialist physician to remove sections with artefacts. Finally, a register free of artefacts, two minutes long, resting state eyes closed was obtained.
2.2 Wavelet Entropy
The Wavelet Entropy is a measure of the degree of order of a signal [1]. Like Shannon entropy, is based in a probability distribution. In this case the distribution of the relative power of a frequency interval with respect to the entire signal power. Therefore, an ordered process could be seen as a very few frequency components that comprises the entire signal power (narrow power spectrum). Then a disordered process contains a several number of frequencies components and has a wide power spectrum. It is expected that in case of cognitive disorder (loss of randomness) the power distribution be less widespread that in case of healthy person. Then the greater the cognitive perturbation, the lower entropy value.
In Wavelet Entropy, the power ratio is calculated by means of the wavelet transform. This is a multi-resolution signal analysis with time-frequency information. As bases functions are used the so called “wavelet functions”. They are short duration, quickly attenuated and zero mean arbitrary functions. The decomposition is based in comparing the signal with the wavelet function displaced in time and for different scales. This leads to coefficients that inform about the degree of similarity of the wavelet with the signal. The use of scales and time translation leads to the more general concept of wavelet family that is represented in (1).
Where \( j,k \) are the scaling and shifting values respectively and \( \psi \) is the wavelet mother.
The original signal \( s\left( t \right) \) can be written as in (2) [18].
Where \( C_{j} \left( k \right) \) is the level \( j \) and scale \( k \) coefficient, \( \psi_{j,k} \left( t \right) \) is the wavelet family and \( DL \) the decomposition level. If (2) is analyzed in the time-frequency domain, the decomposition implementation will be a filter bank of successive high-pass and low-pass filter which provides detail (D) and approximation (A) coefficients respectively (see Fig. 1).
The relative power \( p_{j} \) for the decomposition level \( j \), can be calculated by (3) and the Wavelet Entropy by (4) [18].
Wavelet mother selection has no rules. In general is desired orthogonality and similarity to the analyzed signal. In this work, Symlets9 wavelet mother is used based on the results obtained in [19]. The Haar wavelet mother is used based on its similarity with theta and delta rhythms.
2.3 Data Processing
The EEG artifact-free signal is divided in windows of 400 samples (2 s). Then Wavelet Entropy and EEG rhythms-EEG signal power ratio are calculated for Symlets9 and Haar with five to seven decomposition levels. This values guarantee cover all principal EEG rhythms. The relationship between the decomposition level and each EEG rhythm (frequency) can be calculated using (5) where \( W_{s} \) is the sampling frequency, \( DL \) is the decomposition level and \( \left| W \right| \) is the band pass frequencies. Table 1 shows the relationship.
Once calculated the aforementioned parameter, a statistical analysis is performed using Student’s t-test to determine in which channels and EEG rhythms exists significative differences among the three groups for both wavelets mothers. The objective is to select the wavelet mother who has the greatest number of channels with significative statistical differences and use it for implement a diagnosis methodology.
Entropy is calculated for different time windows. Comparison among groups is mainly made based on its average value [7, 8, 10, 12]. In this work other approach for analyze the time-varying values of entropy is proposed: the maximal difference between entropy histogram peak and its adjacent neighbors (histogram difference hereafter). This is calculated as follow:
-
1.
Calculate the time varying entropy histogram.
-
2.
Normalize the histogram.
-
3.
Select the maximum value of the normalized histogram.
-
4.
Calculate de difference between the maximum value and its right and left neighbors.
-
5.
Select the maximum difference.
-
6.
If the maximum value is the first/last item of the histogram, select the difference with its right/left neighbor.
-
7.
Repeat in all channels of interest.
Once calculated the histogram differences for all channels and both wavelet mothers they are grouped in intervals defined by thresholds with the intention of separate among Healthy, MCI and AD. A proportion test is performed (6) where \( e_{x} \) is the number of people that their histogram difference lies in an interval. \( T_{x} \) is the total number of people in the interval \( x \) [20].
2.4 Evaluated Methodology
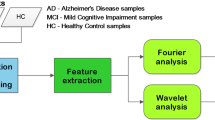
The intended classification methodology is shown in Fig. 2. Once determined the decision characteristics (channel and parameter), they are submitted to the classification block. This is composed of two stages: a pre-classification stage using SVM and the decision stage implemented with neural network. The pre-classification stage is used to reduce the number of inputs of the neural network. The idea is to group channels according to the calculated parameter and their discrimination capability. The neural network present the final decision: Healthy, MCI or AD patients.
3 Results and Discussion
The statistical analysis of EEG rhythm-EEG signal power ratio consider as null hypothesis that Healthy, MCI and AD groups has equal mean but different variance. The alternative hypothesis is that the mean values are different. The results showed that Haar with five decomposition levels and Symlets9 with seven decomposition levels have the best results. In Fig. 3 is shown that Symlets9 detects changes mainly in alpha rhythm while Haar does in beta and theta. Haar also detects changes in a greater number of channels, 14 vs. 9. For this reason, Haar wavelet mother is selected for implement the classification methodology.
The analysis of histogram difference is shown in Fig. 4. The red curve corresponds to AD, blue to MCI and black to Healthy group. In frontal, parietal and central electrodes, the numerical values are different for the three groups so it shows a possible discriminating criterion. The proportion test results are presented in Table 2.
These results allows selecting 27 characteristics to implement the classification methodology. The characteristics are: beta-EEG and theta-EEG power ratio on F3, F4, C3, C4, P4, Fz, Cz, F3, O1; theta-EEG power ratio on F8, C3, P3, T4, T6 and histogram difference on Fp1, Fp2, F3 and Cz. The use of SVM allows reducing the number of inputs of the neural network from 27 to seven. The SVM classifier was tested with polynomial kernel function with order 3 to 10 and multilayer perceptron kernel function with constants of ±1.0 to ±2.0.
The best results were obtained with polynomial kernel function of order 8. The neural network classifier is a two-layer feed-forward network with sigmoid activation function and softmax output neurons with 16 neurons on the hidden layer. It was trained using scaled conjugate gradient backpropagation with 70% of samples for training and 15% for validation and 15% for testing. The data division was random and the test was repeated 20 times averaging results. In Table 3 the sensibility, specificity and accuracy are shown. Figure 5 shows the Receiver Operator Characteristics.
4 Conclusions
It is observed that wavelet decomposition and wavelet entropy have the ability to be used in a classification methodology that allows separating among Healthy, MCI and AD patients using beta-EEG, theta-EEG signal power ratio and the entropy values distribution through time. Haar wavelet mother detects changes in beta and theta rhythms and in a greater number of channels than Symlets9. The latter is sensible mainly to alpha rhythm power changes. The proposed classification methodology has better performance that [8, 13] and comparable with [14] with a greater number of cases. It also has the advantage that classifies among the three possible groups present in a clinical environment: Healthy, MCI and AD people. Its importance lies in the possibility of develop a tool oriented to diagnosis support, which helps to reduce the uncertainty in the diagnosis; especially in MCI where some of the psychological tests fails.
The methodology introduces a new analysis approach for entropy values: histogram analysis of entropy values through time instead the mean value. It also uses the beta-EEG power ratio that has not been used in others studies as far as we know. Is interesting the detection of beta power changes in C3, C4, Fz and Cz in MCI with respect to the healthy group. The reported changes in this rhythm occurs in the posterior regions of the brain in presence of AD [4].
As future work is intended to select the characteristics that more contribute in the classification process in order to reduce the calculation requirements of the processing stage.
References
Alzheimer’s Association: 2017 Alzheimer’s Disease Facts and Figures. Alzheimers Dement, New York (2017)
Alzheimer’ Society: What is Alzheimer’s Disease?, July 2014. https://www.alz.co.uk. Accessed Feb 2016
Bello, V.M.E., Schultz, R.R.: Prevalence of treatable and reversible dementias. Dement Neuropsychol. 5(1), 44–47 (2011)
Al-Qazzaz, N.K., Ali, S.H.B., Ahmad, S.A., Chellappan, K., Islam, M., Escudero, J.: Role of EEG as biomarker in the early detection and classification of dementia. Sci. World J. 2014, 16 (2014)
Wang, R., Wang, J., Li, S., Yu, H., Deng, B., Wei, X.: Multiple feature extraction and classification of electroencephalograph signal for Alzheimer’s with spectrum and bispectrum. Chaos 25, 013110 (2015)
Mittal, S.H., et al.: Abnormal levels of consciousness and their electroencephalogram correlation: a review. EC Neurol. 4(1), 30–35 (2016)
Abásolo, D., Hornero, R., Espino, P., Alvarez, D., Poza, J.: Entropy analysis of the EEG background activity in Alzheimer’s disease patients. Physiol. Meas. 27, 241–253 (2009)
Hornero, R., Abásolo, D., Escudero, J., Gómez, C.: Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer’s disease. Phil. Trans. R. Soc. 367, 317–336 (2009)
De Bock, T.J., et al.: Early detection of Alzheimer’s disease using nonlinear analysis of EEG via Tsallis entropy. In: Biomedical Sciences and Engineering Conference (2010)
Al-nuaimi, A.H., Jammeh, E., Sun, L., Ifeachor, E.: Tsallis entropy as a biomarker for detection of Alzheimer’s disease. In: 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (2015)
Escudero, J., Acar, E., Fernández, A., Bro, R.: Multiscale entropy analysis of resting-state magneto encephalogram with tensor factorizations in Alzheimer’s disease. Brain Res. Bull. 119(Part B), 136–144 (2015)
Morabito, F.C., Labate, D., La Foresta, F., Bramanti, A., Morabito, G., Palamara, I.: Multivariate multi-scale permutation entropy for complexity analysis of Alzheimer’s disease EEG. Entropy 14, 1186–1202 (2012)
Simons, S., Espino, P., Abásolo, D.: Fuzzy entropy analysis of the electroencephalogram in patients with Alzheimer’s disease: is the method superior to sample entropy? Entropy 20(21), 13 (2018)
Ghorbanian, P., Devilbiss, D.M., Hess, T., Bernstein, A., Simon, A.J., Ashrafiuon, H.: Exploration of EEG features of Alzheimer’s disease using continuous wavelet transform. Med. Biol. Eng. Comput. 53, 843–855 (2015)
Edmonds, E.C., et al.: Susceptibility of the conventional criteria for mild cognitive impairment. Alzheimer’s Dementia 11, 1–10 (2014)
Charernboon, T.: Diagnostic accuracy of the overlapping infinity loops, wire cube, and clock drawing tests for cognitive impairment and dementia. Int. J. Alzheimer’s Dis. 2017, 5 (2017)
Allan, C.L., Behrman, S., Ebmeier, K.P., Valkanova, V.: Diagnosing early cognitive decline—when, how and for whom? Maturitas 2017, 103–108 (2017)
Rosso, O.A., et al.: Wavelet entropy: a new tool for short duration brain electrical signals. J. Neurosci. Methods 105, 65–75 (2001)
Al-Qazzaz, N., Hamid Bin Mohd Ali, S., Ahmad, S., Islam, M., Escudero, J.: Selection of mother wavelet functions for multi-channel EEG signal analysis during a working memory task. Sensors 15, 21 (2015)
Castillo, A.J.S.: Apuntes de Estadística para Ingenieros. Creative Commons, Jaén (2012)
Jeong, D.H., Kim, Y.D., Song, I.U., Chung, Y.A., Jeong, J.: Wavelet energy and wavelet coherence as EEG biomarkers for the diagnosis of Parkinson’s disease-related dementia and Alzheimer’s disease. Entropy 18(8), 17 (2015)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Toural, J.E.S., Pedrón, A.M., Marañón, E.J. (2019). A Wavelet Entropy Based Methodology for Classification Among Healthy, Mild Cognitive Impairment and Alzheimer’s Disease People. In: Nyström, I., Hernández Heredia, Y., Milián Núñez, V. (eds) Progress in Pattern Recognition, Image Analysis, Computer Vision, and Applications. CIARP 2019. Lecture Notes in Computer Science(), vol 11896. Springer, Cham. https://doi.org/10.1007/978-3-030-33904-3_55
Download citation
DOI: https://doi.org/10.1007/978-3-030-33904-3_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33903-6
Online ISBN: 978-3-030-33904-3
eBook Packages: Computer ScienceComputer Science (R0)