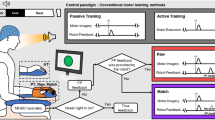
Abstract
Feedback is an important part of the BCI loop providing a user with the information regarding whether or not cognitive efforts were successful. It is believed that the feedback affects user’s learning ability to control BCI. In the field of neurorehabilitation, the feedback of a BCI loop is associated with facilitation of neuroplasticity. However, such methods as motor imagery, tactile stimulation and movements observation have a therapeutic effect per se which makes it unclear whether any facilitation is associated with a BCI loop. The aim of the present study is to fill such a lacuna of knowledge. The answer can help us to propose a new paradigm of neurorehabilitation instruments to recover the mobility of patients paralyzed after a stroke. P300-BCI is another popular paradigm which is based on visual evoked potentials and being commonly used in BCI spellers. In our study, we examined whether the feedback in P300-BCI loop could facilitate motor cortex activity. We have shown that primary motor cortex TMS-induced MEPs was higher in amplitude for sensory information perceived as a feedback than for perceived per se, while the MO and TS per se did not affect the excitability of the motor cortex. We conclude that the BCI-feedback context influenced the perception of visual and tactile information. These results can be used to build a new generation of BCI for rehabilitation since it is known that the ability to control P300-BCI for post-stroke patients remains at a high level.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Activation of the motor control cortical systems by means of motor imagery is widely considered as one of the most promising approaches for motor rehabilitation [1]. A series of studies based on transcranial magnetic stimulation (TMS) technique showed a significant increase in corticospinal excitability during motor imagery, which allows us to consider it as a relevant approach for motor rehabilitation [2, 3]. Feedback is known to be the key condition for mental training since it provides a subject with information regarding the quality of any motor imagery act. So, it is important to note that any BCI loop involves a feedback perception. Currently, BCIs based on motor imagery are actively used to create neurorehabilitation tools and techniques aimed at motor recovery of patients paralyzed after a stroke [4]. Such tools allow patients not only to train a paralyzed limb but also to control exoskeletal structures that move paralyzed parts of the body, which may enhance training effects [4]. However, an MI-BCI does not allow to generate more than 2–4 feedback signals or commands for an external device. This limitation is present due to a limited set of mental images, which might be detected through EEG mu-rhythm desynchronization. Moreover, the classification accuracy for such BCIs frequently cannot exceed 65–75% [5]. Also, to operate an MI-based BCI a post-stroke patient must undergo a long training session whereas their overall ability to imagine movement may be impaired after a stroke [6].
In conformity with the facts mentioned, the BCI community tends to develop reliable and multitasking interfaces to be used in neurorehabilitation. Amongst all the existing solutions, the interfaces based on a positive wave of visual evoked potential P300 (P300-BCI) are known to be the most appropriate. An important argument for such a claim is that post-stroke patients can quickly master this BCI technology [7]. Moreover, the accuracy of mental command classification may reach 80–90% whereas the number of commands is limited only by the number of on-screen stimuli denoting these commands. However, the activity of a subject in a P300-BCI does explicitly include neither motor imagery nor movements itself. Therefore, it might be questioned whether the P300-based BCIs can be used as a neurorehabilitation instrument to recover the mobility of patients paralyzed after a stroke. The present study aims to test the hypothesis that the operation of a P300-BCI by a subject (specifically, control of the finger movements of an artificial hand and the vibrotactile stimulation of their own hand) leads to an increase in corticospinal excitability. This assumption is based on the fact that observation of artificial hand finger movements as feedback can activate the primary motor cortex of an observing subject. After a lot of studies in the field have been published, the fact that both movement observation and tactile stimulation can lead to a change in the activity of the motor cortex is considered as common knowledge. Feedback is an important part of a BCI loop as it provides an operator with the information considering the degree of success of their cognitive efforts. It is well known that the presence of feedback affects the ability of a subject to operate in a BCI loop. More than that, in [8] it was shown that vibrotactile feedback is more useful for some subjects than visual one while learning to operate an MI-based BCI. Also, in [9] the authors showed that the location of a vibrating motor during tactile feedback perception affects BCI performance. At the same time, it remains unknown whether any advantage of BCI loop implementation for neurorehabilitation exists. One more question which keeps being unanswered is whether the feedback-associated sensation (the sensory input perceived as a response to the mental efforts of a subject) affects the perception of sensory information? The answer to this question was obtained in this study which allowed us to make some recommendations regarding the development of motor recovery tools and techniques based on P300-BCI.
2 Methods
2.1 Participants
The study involved 20 healthy volunteers (7 women) aged 20 to 26 years. All of them signed an informed consent to participate in a study approved by the Department of Biology of Moscow University.
2.2 Protocol
P300-BCI.
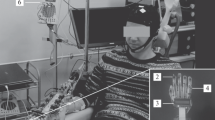
An artificial five-fingered limb similar to a human hand was used in the experiment as a tool for stimuli presentation and visual feedback output. It was located above the right hand of a subject. Each finger of the artificial limb is movable through the P300-BCI. A light-emitting diode (LED) was mounted on each finger of the artificial limb. Random alternate illumination of the five LEDs served as stimuli to evoke an EEG response which is essential for the extraction of P300 component. The subject selected a finger of the artificial hand which they would like to flex and concentrated their visual attention on the illumination of a corresponding LED. Visual evoked potentials that were recorded in response to flashes of both targeted and non-targeted stimuli (LEDs on fingers) were used for classification of the intention of a subject (regarding which finger to flex). After the finished classification process, a subject received feedback as either: 1 - observation of selected artificial finger movement - MO(bci), 2 - observation of selected artificial finger movement + vibrotactile stimulation of the corresponding right-hand finger - MO + T (bci), 3 - vibrotactile stimulation of the corresponding right-hand finger - T (bci) Also, we introduced two additional conditions to be used as control measures: 4 - vibrotactile stimulation of a random finger (perceived as a sensory signal with no BCI-feedback properties) - T (non bci), 5 - observation of a random artificial finger movement - MO (non bci). The electroencephalogram was recorded with a sampling frequency of 500 Hz from Pz, Poz, 28 PO3, PO4, PO7, PO8, O1, O2 sites (according to the “10–10” system). ERPs recorded in response to the illumination of targeted and non-targeted LED flashes were classified using LDA. The average classification accuracy for all the subjects was 95% of the total trial number (20 trials).
Transcranial Magnetic Stimulation (TMS) and Electromyography (EMG).
Transcranial magnetic stimulation is a technique for noninvasive stimulation of the human brain. Single pulse transcranial magnetic stimuli were delivered using a Neurosoft stimulator (Ivanovo, Rus), via a figure-of-eight coil. The coil was positioned over the left motor cortex, at the optimal site for producing responses in the resting flexor digitorum superficialis muscle (FDS) with 110% amplitude (according to motor threshold). Electromyography was recorded to determine the amplitude of motor-evoked potentials (MEPs). The change in the amplitude of the MEP is known to reflects the dynamics of corticospinal excitability of a subject.
3 Results and Discussion
Analysis of MEP amplitudes showed that in all the conditions in which a subject perceived any kind of BCI feedback, corticospinal excitability significantly increased. Accordingly, the perception of feedback-associated sensory information (motor observation and vibrotactile stimulation) led to an increase of MEPs amplitude (see Fig. 1). However, it is unclear whether such an increase is connected with the BCI operating since it is known that motor observation per se may activate M1 [10]. To resolve such a problem, in all of the non-BCI conditions motor observation was set passive (non-BCI induced) as well as vibrotactile stimulation was performed arbitrarily. In turn, MEPs amplitude in non-BCI conditions did not change after the perception of sensory information. More than that, in the condition of non-BCI motor observation, MEP amplitudes recorded after observation, were significantly lower than the ones recorded in MO-BCI condition. The same trend is present for tactile stimulation as well, i.e. the perception of a tactile stimulus increased corticospinal excitability only if such a stimulus was associated with BCI feedback (see Fig. 1).
Amplitude of motor-evoked potentials (MEP) for FDS of all subjects regarding to different types of feedback. MO corresponds to MEPs which were recorded during observation of movement of target finger, MO + T - corresponds to combined condition (observation of movement + tactile stimulation). T – corresponds to the cases when participants perceived tactile stimulation of their own fingers. Postfix «bci» denotes conditions when sensory information was presented as a BCI feedback Amplitude of responses is presented as a percentage of the baseline condition mean MEPs. Columns and error bars are median and interquartile range of distribution of each group of MEPs. Note: comparison with the baseline - #p < 0,05/comparison between conditions - *p < 0,05 (Mann-Whitney test, p-values were adjusted by Dunn’s test).
Thus, we can conclude that the perception of visual and tactile sensory information is influenced by the feedback context in the P300-BCI loop. According to the MEP amplitude analysis, passive observation of movement (outside of the BCI loop) did not increase corticospinal excitability. Probably, such a result is caused by insufficient anthropomorphism of the artificial hand. The perception of tactile stimulation outside of the BCI loop led to insignificantly decreased MEP amplitudes. We suggest that either random fluctuations or short-latency afferent inhibition have led to such a minor decrease [11]. Also, we do not exclude «bottom-up» activation of M1 in the BCI conditions, which might occur through afferent neural pathways and cortico-cortical connections between M1 and visual cortex [12]. However, we claim that the key factor for the modulation observed as «top-down» interaction since the detection of feedback-associated components in sensory input is essential for the process according to the results we obtained.
4 Conclusions
We conclude that P300-based BCIs can be used in neurorehabilitation. Such BCIs are considered as user-friendly for post-stroke patients and allows them to perform control with high accuracy. We claim that during the construction of neurorehabilitation devices it is essential to take into account the presence of the feedback and its sensory modality since the perception of feedback in the P300-BCI loop modulates activation of motor cortex according to the results of the study. Moreover, we find it worth noting that all the experiments in the study are conducted on healthy subjects. Accordingly, before the implementation of the technique for after-stroke patients the individual differences and clinical pictures of the patients are to be taken into account to choose and use the feedback of the most effective for particular patient sensory modality.
References
Dijkerman, H.C., Ietswaart, M., Johnston, M.: Motor imagery and the rehabilitation of movement disorders: an overview. Neurophysiological Found. Ment. Motor Imagery 1(9), 127–144 (2010)
Kaplan, A., Vasilyev, A., Liburkina, S.: Poor BCI performers still could benefit from motor imagery training. In: International Conference on Augmented Cognition, pp. 46–56 (2016)
Yakovlev, L.V., Syrov, N.V., Kaplan, A.Y.: Corticospinal excitability in humans during motor imagery coupled with functional electrical stimulation. Moscow Univ. Biol. Sci. Bull. 74(3), 183–187 (2019)
Ang, K.K., Guan, C., Zhang, H.: Clinical study of neurorehabilitation in stroke using EEG-based motor imagery brain-computer interface with robotic feedback. In: Annual International Conference of the IEEE Engineering in Medicine and Biology 2010, pp. 5549–5552. IEEE (2010)
Ahn, M., Jun, S.C.: Performance variation in motor imagery brain–computer interface: a brief review. J. Neurosci. Methods 243, 103–110 (2015)
Malouin, F., Richards, C.L., Durand, A.: Clinical assessment of motor imagery after stroke. Neurorehab. Neural Repair 22(4), 330–340 (2008)
Ortner, R., Bruckner, M., Prueckl, R.: Accuracy of a P300 speller for people with motor impairments. In: 2011 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), pp. 1–6, April 2011
Liburkina, S.P., Vasilyev, A.N., Yakovlev, L.: Motor imagery-based brain-computer interface with vibrotactile stimuli. Neurosci. Behav. Physiol. 48(9), 1067–1077 (2018)
Rahman, K.A.A., Ibrahim, B.S.K.K., Fuad, N.: ACIS advances in computing and intelligent system Brain Computer Interface (BCI) - Functional Electrical Stimulation (FES) control system of knee joint movement for paraplegic. Adv. Comput. Intell. Syst. 1(1), 1–6 (2019)
Zhang, J.J., Fong, K.N., Welage, N.: The activation of the mirror neuron system during action observation and action execution with mirror visual feedback in stroke: a systematic review. Neural Plast. 2018, 10–14 (2018)
Turco, C.V., El-Sayes, J., Savoie, M.J.: Short-and long-latency afferent inhibition; uses, mechanisms and influencing factors. Brain Stimul. 11(1), 59–74 (2018)
Robertson, I.H., Murre, J.M.: Rehabilitation of brain damage: brain plasticity and principles of guided recovery. Psychol. Bull. 125(5), 544 (1999)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Syrov, N., Bredichin, D., Kaplan, A. (2020). Processing of Sensory Information is Affected by BCI Feedback Being Perceived. In: Stephanidis, C., Antona, M. (eds) HCI International 2020 - Posters. HCII 2020. Communications in Computer and Information Science, vol 1224. Springer, Cham. https://doi.org/10.1007/978-3-030-50726-8_75
Download citation
DOI: https://doi.org/10.1007/978-3-030-50726-8_75
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50725-1
Online ISBN: 978-3-030-50726-8
eBook Packages: Computer ScienceComputer Science (R0)