Abstract
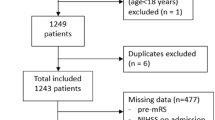
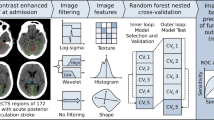
Prognosis in Wake-up ischemic stroke (WUS) is important for guiding treatment and rehabilitation strategies, in order to improve recovery and minimize disability. For this reason, there is growing interest on models to predict functional recovery after acute ischemic events in order to personalize the therapeutic intervention and improve the final functional outcome. The aim of this preliminary study is to evaluate the possibility to predict a good functional outcome, in terms of modified Rankin Scale (mRS ≤ 2), in thrombolysis treated WUS patients by Bayesian analysis of clinical, demographic and neuroimaging data at admission. The study was conducted on 54 thrombolysis treated WUS patients. The Variational Bayesian logistic regression with Automatic Relevance Determination (VB-ARD) was used to produce model and select informative features to predict a good functional outcome (mRS ≤ 2) at discharge. The produced model showed moderately high 10 × 5-fold cross validation accuracy of 71% to predict outcome. The sparse model highlighted the relevance of NIHSS at admission, age, TACI stroke syndrome, ASPECTs, ischemic core CT Perfusion volume, hypertension and diabetes mellitus. In conclusion, in this preliminary study we assess the possibility to model the prognosis in thrombolysis treated WUS patients by using VB ARD. The identified features related to initial neurological deficit, history of diabetes and hypertension, together with necrotic tissue relate ASPECT and CTP core volume neuroimaging features, were able to predict outcome with moderately high accuracy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gorelick, P.B.: The global burden of stroke: persistent and disabling. Lancet Neurol. 18(5), 417–418 (2019)
Mackey, J., Kleindorfer, D., Sucharew, H., et al.: Population-based study of wake-up strokes. Neurology 76, 1662–1667 (2011)
Thomalla, G., Fiebach, J.B., Ostergaard, L., et al.: A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int. J. Stroke 9, 829–836. https://doi.org/10.1111/ijs.12011
Vilela, P., Rowley, H.A.: Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur. J. Radiol. 96, 162–172 (2017)
Furlanis, G., et al.: Wake-up stroke: thrombolysis reduces ischemic lesion volume and neurological deficit. J. Neurol. 267(3), 666–673 (2019). https://doi.org/10.1007/s00415-019-09603-7
Caruso, P., et al.: Wake-up stroke and CT perfusion: effectiveness and safety of reperfusion therapy. Neurol. Sci. 39(10), 1705–1712 (2018). https://doi.org/10.1007/s10072-018-3486-z
Peisker, T., Koznar, B., Stetkarova, I., et al.: Acute stroke therapy: a review. Trends Cardiovasc. Med. 27, 59–66 (2017)
Stragapede, L., Furlanis, G., Ajčević, M., et al.: Brain oscillatory activity and CT perfusion in hyper-acute ischemic stroke. J. Clin. Neurosci. 69, 184–189 (2019). https://doi.org/10.1016/j.jocn.2019.07.068
Ma, H., Campbell, B.C.V., Parsons, M.W., et al.: Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N. Engl. J. Med. 380, 1795–1803 (2019). https://doi.org/10.1056/NEJMoa1813046
Bentes, C., Peralta, A.R., Viana, P., et al.: Quantitative EEG and functional outcome following acute ischemic stroke. Clin. Neurophysiol. 129(8), 1680–1687 (2018)
Banks, J.L., Marotta, C.A.: Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 38(3), 1091–1096 (2007)
Caruso, P., Ajčević, M., Furlanis, G., et al.: Thrombolysis safety and effectiveness in acute ischemic stroke patients with pre-morbid disability. J. Clin. Neurosci. 72, 180–184 (2020). https://doi.org/10.1016/j.jocn.2019.11.047
Saver, J.L., Filip, B., Hamilton, S., et al.: Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke 41(5), 992–995 (2010)
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333, 1581–1588 (1995)
Bamford, J., Sandercock, P., Dennis, M., et al.: Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337(8756), 1521–1526 (1991)
Adams Jr., H.P., Davis, P.H., Leira, E.C., et al.: Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53, 126–131 (1999). https://doi.org/10.1212/wnl.53.1.126
Barber, P.A., Demchuk, A.M., Zhang, J., Buchan, A.M., for the ASPECTS Study Group: The validity and reliability of a novel quantitative CT score in predicting outcome in hyperacute stroke prior to thrombolytic therapy. Lancet 355, 1670–1674 (2000)
Furlanis, G., Ajčević, M., Stragapede, L., et al.: Ischemic volume and neurological deficit: correlation of computed tomography perfusion with the National Institutes of Health Stroke Scale Score in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 27(8), 2200–2207 (2018). https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.04.003
Granato, A., D’Acunto, L., Ajčević, M., et al.: A novel Computed Tomography Perfusion-based quantitative tool for evaluation of perfusional abnormalities in migrainous aura stroke mimic. Neurol. Sci. (2020). https://doi.org/10.1007/s10072-020-04476-5
Wintermark, M., Flanders, A.E., Velthuis, B., et al.: Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 37, 979–985 (2006)
Treder, M.S., Blankertz, B.: (C)overt attention and visual speller design in an ERP-based brain-computer interface. Behav. Brain Funct. (2010). https://doi.org/10.1186/1744-9081-6-28
Drugowitsch, J.: Variational Bayesian inference for linear and logistic regression. arXiv e-prints (2013)
Bishop, C.M.: Pattern Recognition and Machine Learning. Springer, New York (2006)
MacKay, D.J.C.: Bayesian interpolation. Neural Comput. 4(3), 415–447 (1992)
Neal, R.M.: Bayesian Learning for Neural Networks. Springer, New York (1996). https://doi.org/10.1007/978-1-4612-0745-0
Tipping, M.E.: Sparse Bayesian learning and the relevance vector machine. J. Mach. Learn. Res. 1, 211–244 (2001)
Jaakkola, T.S., Jordan, M.M.: Bayesian parameter estimation via variational methods. Stat. Comput. 10, 25–37 (2000). https://doi.org/10.1023/A:1008932416310
Zikopoulos, P., Eaton, C.: Understanding Big Data Analytics for Enterprise Class Hadoop and Streaming Data. McGraw Hill, New York (2012)
Cuzzocrea, A., Moussa, R., Xu, G.: OLAP*: effectively and efficiently supporting parallel OLAP over big data. In: Cuzzocrea, A., Maabout, S. (eds.) MEDI 2013. LNCS, vol. 8216, pp. 38–49. Springer, Heidelberg (2013). https://doi.org/10.1007/978-3-642-41366-7_4
Chatzimilioudis, G., Cuzzocrea, A., Gunopulos, D., Mamoulis, N.: A novel distributed framework for optimizing query routing trees in wireless sensor networks via optimal operator placement. J. Comput. Syst. Sci. 79(3), 349–368 (2013)
Cuzzocrea, A.: Combining multidimensional user models and knowledge representation and management techniques for making web services knowledge-aware. Web Intell. Agent Syst. 4(3), 289–312 (2006)
Cuzzocrea, A., Bertino, E.: Privacy preserving OLAP over distributed XML data: a theoretically-sound secure-multiparty-computation approach. J. Comput. Syst. Sci. 77(6), 965–987 (2011)
Cuzzocrea, A., Russo, V.: Privacy preserving OLAP and OLAP security. In: Encyclopedia of Data Warehousing and Mining, pp. 1575–1581 (2009)
Wang, L., Alexander, C.A.: Stroke care and the role of big data in healthcare and stroke. Rehabil. Sci. 1(1), 16–24 (2016)
Nishimura, A., Nishimura, K., Kada, A., Iihara, K., J-ASPECT Study Group: Status and future perspectives of utilizing big data in neurosurgical and stroke research. Neurol. Med.-Chir. 56(11), 655–663 (2016)
Burke Quinlan, E., Dodakian, L., See, J., et al.: Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann. Neurol. 77, 132–145 (2015)
Spyroglou, I.I., Spöck, G., Chatzimichail, E.A., et al.: A Bayesian logistic regression approach in asthma persistence prediction. Epidemiol. Biostat. Public Health 15(1), e12777 (2018)
Ashby, D.: Bayesian statistics in medicine: a 25 year review. Stat. Med. 25(21), 3589–3631 (2006)
Miladinović, A., et al.: Slow cortical potential BCI classification using sparse variational Bayesian logistic regression with automatic relevance determination. In: Henriques, J., Neves, N., de Carvalho, P. (eds.) MEDICON 2019. IP, vol. 76, pp. 1853–1860. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-31635-8_225
Weimar, C., König, I.R., Kraywinkel, K., et al.: Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35, 158–162 (2004)
Saver, J.L., Altman, H.: Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke 43, 1537–1541 (2012)
Di Carlo, A., Lamassa, M., Baldereschi, M., et al.: Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. The European Community Stroke Project. J. Neurol. Sci. 244, 143–150 (2006)
Desilles, J.P., Meseguer, E., Labreuche, J., et al.: Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 44, 1915–1923 (2013)
Manabe, Y., Kono, S., Tanaka, T., et al.: High blood pressure in acute ischemic stroke and clinical outcome. Neurol. Int. 1(1), e1 (2009). https://doi.org/10.4081/ni.2009.e1
Baek, J.H., Kim, K., Lee, Y.B., et al.: Predicting stroke outcome using clinical- versus imaging-based scoring system. J. Stroke Cerebrovasc. Dis. 24(3), 642–648 (2015). https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.10.009
Bivard, A., Spratt, N., Miteff, F., et al.: Tissue is more important than time in stroke patients being assessed for thrombolysis. Front. Neurol. 9, 41 (2018)
Tian, H., Parsons, M.W., Levi, C.R., et al.: Influence of occlusion site and baseline ischemic core on outcome in patients with ischemic stroke. Neurology 92, e2626–e2643 (2019). https://doi.org/10.1212/WNL.0000000000007553
Ajčević, M., Furlanis, G., Buoite Stella, A., et al.: CTP based model predicts outcome in rTPA treated wake-up stroke patients. Physiol. Meas. (2020). https://doi.org/10.1088/1361-6579/ab9c70
Acknowledgements
This study was partially supported by Master in Clinical Engineering, University of Trieste. A. Miladinović is supported by the European Social Fund (ESF) and Autonomous Region of Friuli Venezia Giulia (FVG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflict of interest do declare.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Ajčević, M. et al. (2020). A Big-Data Variational Bayesian Framework for Supporting the Prediction of Functional Outcomes in Wake-Up Stroke Patients. In: Gervasi, O., et al. Computational Science and Its Applications – ICCSA 2020. ICCSA 2020. Lecture Notes in Computer Science(), vol 12249. Springer, Cham. https://doi.org/10.1007/978-3-030-58799-4_71
Download citation
DOI: https://doi.org/10.1007/978-3-030-58799-4_71
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58798-7
Online ISBN: 978-3-030-58799-4
eBook Packages: Computer ScienceComputer Science (R0)