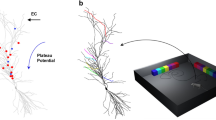
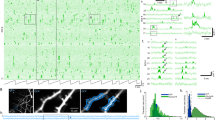
Abstract
Hippocampal Place Cells play a pivotal role in spatial navigation. These cells have the particular characteristic of firing at a low rate during navigation throughout most of the environment, except when the animal is within a restricted spatial region called place field. The biophysical mechanisms underlying their formation or remapping following external sensory inputs are poorly understood. Recent experimental evidence clearly showed that, in the CA1 hippocampal region, the interaction between a properly timed association of inputs from the entorhinal cortex and the CA3 region can induce a novel place field formation. On CA1 pyramidal neurons, these different inputs are spatially segregated: the input from entorhinal cortex targets the most distal apical dendritic regions, while the CA3 input arrives onto proximal dendrites. The conditions under which this interaction can explain the formation of a place field in a CA1 pyramidal neuron are not fully understood. In this work, we present a series of simulations using a morphologically and biophysically detailed model of a CA1 pyramidal neuron. We tested the model by simulating a mouse random spatial navigation in a small room with objects. Following a reward signal activated during the navigation in the distal dendrites, as a forward traveling depolarization envelope, the neuron was able to selectively potentiate only the synapses coding for the object present in the visual field. Subsequent navigation through the same environment resulted in the neuron firing as expected for a place cell.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Burgess, N., O’Keefe, J.: Neuronal computations underlying the firing of place cells and their role in navigation. Hippocampus 6, 749–762 (1996). https://doi.org/10.1002/(sici)1098-1063(1996)6:6%3c749::aid-hipo16%3e3.0.co;2-0
O’Keefe, J., Dostrovsky, J.: The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). https://doi.org/10.1016/0006-8993(71)90358-1
Fox, S.E., Ranck, J.B.: Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp Neurol. 49, 299–313 (1975). https://doi.org/10.1016/0014-4886(75)90213-7
Hazama, Y., Tamura, R.: Effects of self-locomotion on the activity of place cells in the hippocampus of a freely behaving monkey. Neurosci Lett. 701, 32–37 (2019). https://doi.org/10.1016/J.NEULET.2019.02.009
Hazama, Y., Tamura, R.: Data on the activity of place cells in the hippocampal CA1 subfield of a monkey performing a shuttling task. Data Brief. 26 (2019). https://doi.org/10.1016/J.DIB.2019.104467
Ekstrom, A.D., et al.: Cellular networks underlying human spatial navigation. Nature 425, 184–187 (2003). https://doi.org/10.1038/NATURE01964
O’Keefe, J., Nadel, L.: The Hippocampus as a Cognitive Map. Clarendon Press, Oxford (1978)
Wilson, M.A., McNaughton, B.L.: Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 (1993). https://doi.org/10.1126/SCIENCE.8351520
Alme, C.B., Miao, C., Jezek, K., Treves, A., Moser, E.I., Moser, M.B.: Place cells in the hippocampus: eleven maps for eleven rooms. Proc. Natl. Acad. Sci. U S A. 111, 18428–18435 (2014). https://doi.org/10.1073/PNAS.1421056111
Muller, R.U., Kubie, J.L.: The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968 (1987). https://doi.org/10.1523/JNEUROSCI.07-07-01951.1987
Fenton, A.A., Csizmadia, G., Muller, R.U.: Conjoint control of hippocampal place cell firing by two visual stimuli. I. The effects of moving the stimuli on firing field positions. J. Gen. Physiol. 116, 191–209 (2000). https://doi.org/10.1085/JGP.116.2.191
Sharp, P.E.: Computer simulation of hippocampal place cells. Psychobiology 19(2), 103–115 (2013). https://doi.org/10.3758/BF03327179
Zipser, D.: Biologically plausible models of place recognition and goal location. In: McClelland, J.L., Rumelhart, D.E., PDP Research Group (eds.) Parallel Distributed Processing: Explorations in the Microstructure of Cognition, vol. 2, pp. 432–470. MIT Press, Cambridge (1986)
Bittner, K.C., et al.: Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 18, 1133–1142 (2015). https://doi.org/10.1038/NN.4062
Migliore, M., Novara, G., Tegolo, D.: Single neuron binding properties and the magical number 7. Hippocampus 18, 1122–1130 (2008). https://doi.org/10.1002/HIPO.20480
Migliore, M., Cannia, C., Canavier, C.C.: A modeling study suggesting a possible pharmacological target to mitigate the effects of ethanol on reward-related dopaminergic signaling. J. Neurophysiol. 99, 2703–2707 (2008). https://doi.org/10.1152/JN.00024.2008
Hoffman, D.A., Magee, J.C., Colbert, C.M., Johnston, D.: K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875 (1997). https://doi.org/10.1038/43119
Magee, J.C.: Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. 18, 7613–7624 (1998). https://doi.org/10.1523/JNEUROSCI.18-19-07613.1998
Migliore, M.: On the integration of subthreshold inputs from Perforant Path and Schaffer Collaterals in hippocampal CA1 pyramidal neurons. J Comput. Neurosci. 14, 185–192 (2003). https://doi.org/10.1023/A:1021906818333
Gasparini, S., Migliore, M., Magee, J.C.: On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J. Neurosci. 24, 11046–11056 (2004). https://doi.org/10.1523/JNEUROSCI.2520-04.2004
Bianchi, D., et al.: Effects of increasing CREB-dependent transcription on the storage and recall processes in a hippocampal CA1 microcircuit. Hippocampus 24, 165–177 (2014). https://doi.org/10.1002/HIPO.22212
Quiroga, R.Q., Reddy, L., Kreiman, G., Koch, C., Fried, I.: Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107 (2005). https://doi.org/10.1038/NATURE03687
Fried, I., MacDonald, K.A., Wilson, C.L.: Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 18, 753–765 (1997). https://doi.org/10.1016/S0896-6273(00)80315-3
Kornblith, S., Quian Quiroga, R., Koch, C., Fried, I., Mormann, F.: Persistent single-neuron activity during working memory in the human medial temporal lobe. Curr. Biol. 27, 1026–1032 (2017). https://doi.org/10.1016/J.CUB.2017.02.013
Migliore, M., de Blasi, I., Tegolo, D., Migliore, R.: A modeling study suggesting how a reduction in the context-dependent input on CA1 pyramidal neurons could generate schizophrenic behavior. Neural. Netw. 24, 552–559 (2011). https://doi.org/10.1016/J.NEUNET.2011.01.001
Kastellakis, G., Silva, A.J., Poirazi, P.: Linking memories across time via neuronal and dendritic overlaps in model neurons with active dendrites. Cell Rep. 17, 1491–1504 (2016). https://doi.org/10.1016/J.CELREP.2016.10.015
Lisman, J.: Working memory: the importance of theta and gamma oscillations. Curr Biol. 20 (2010). https://doi.org/10.1016/J.CUB.2010.04.011
Fuentemilla, L., Penny, W.D., Cashdollar, N., Bunzeck, N., Düzel, E.: Theta-coupled periodic replay in working memory. Curr. Biol. 20, 606–612 (2010). https://doi.org/10.1016/J.CUB.2010.01.057
Jensen, O., Kaiser, J., Lachaux, J.P.: Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324 (2007). https://doi.org/10.1016/J.TINS.2007.05.001
Gray, C.M.: Synchronous oscillations in neuronal systems: mechanisms and functions. J. Comput. Neurosci. 1, 11–38 (1994). https://doi.org/10.1007/BF00962716
Hartley, T., Burgess, N., Lever, C., Cacucci, F., O’Keefe, J.: Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379 (2000). https://doi.org/10.1002/1098-1063(2000)10:4%3c369::aid-hipo3%3e3.0.co;2-0
Barry, C., Burgess, N.: Learning in a geometric model of place cell firing. Hippocampus 17, 786–800 (2007). https://doi.org/10.1002/HIPO.20324
Yoder, R.M., Clark, B.J., Taube, J.S.: Origins of landmark encoding in the brain. Trends Neurosci. 34, 561–571 (2011). https://doi.org/10.1016/J.TINS.2011.08.004
Knierim, J.J., Rao, G.: Distal landmarks and hippocampal place cells: effects of relative translation versus rotation. Hippocampus 13, 604–617 (2003). https://doi.org/10.1002/HIPO.10092
Knierim, J.J., Kudrimoti, H.S., McNaughton, B.L.: Place cells, head direction cells, and the learning of landmark stability. J. Neurosci. 15, 1648–1659 (1995). https://doi.org/10.1523/JNEUROSCI.15-03-01648.1995
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Mazzara, C., Comelli, A., Migliore, M. (2022). Place Cell’s Computational Model. In: Mazzeo, P.L., Frontoni, E., Sclaroff, S., Distante, C. (eds) Image Analysis and Processing. ICIAP 2022 Workshops. ICIAP 2022. Lecture Notes in Computer Science, vol 13373. Springer, Cham. https://doi.org/10.1007/978-3-031-13321-3_35
Download citation
DOI: https://doi.org/10.1007/978-3-031-13321-3_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13320-6
Online ISBN: 978-3-031-13321-3
eBook Packages: Computer ScienceComputer Science (R0)