Abstract
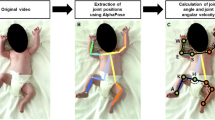
Preterm infants’ spontaneous movements monitoring is a valuable ally to early recognise neuro-motor impairments, especially common in infants born before term. Currently, highly-specialized clinicians assess the movements quality on the basis of subjective, discontinuous, and time-consuming observations. To support clinicians, automatic monitoring systems have been developed, among which Deep Learning algorithms (mainly Convolutional Neural Networks (CNNs)) are up-to-date the most suitable and less invasive ones. Indeed, research in this field has devised highly reliable models, but has shown a tendency to neglect their computational costs. In fact, these models usually require massive computations, which, in turn, require expensive hardware and are environmentally unsustainable. As a consequence, the costs of these models risk to make their application to the actual clinical practice a privilege. However, the ultimate goal of research, especially in healthcare, should be designing technologies that are fairly accessible to as many people as possible. In light of this, this work analyzes three CNNs for preterm infants’ movements monitoring on the basis of their computational requirements. The two best-performing networks achieve very similar accuracy (Dice Similarity Coefficient around 0.88) although one of them, which was designed by us following the principles of Green AI, requires half as many Floating Point Operations (\(47\times 10^9\) vs \(101\times 10^9\)). Our research show that it is possible to design highly-performing and cost-efficient Convolutional Neural Networks for clinical applications .
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
- 2.
- 3.
Throughout the paper, the word “fairness” will always refer to distributive fairness in the discussed algorithms. To expand different dimensions of fairness that can be promoted via AI technology, see G. Tiribelli (2022) [6].
- 4.
The FLOPs were computed with a dedicated Python package, available at https://github.com/tokusumi/keras-flops.
- 5.
Model compression can be applied on any Artificial Neural Network, but for the sake of consistency we only refer CNNs.
References
Agency, I.E.: Key world energy statistics 2021. IEA, Paris (2021). https://www.iea.org/reports/key-world-energy-statistics-2021
Anum, E.A., Springel, E.H., Shriver, M.D., Strauss, J.F.: Genetic contributions to disparities in preterm birth. Pediatr. Res. 65(1), 1–9 (2009)
Buciluǎ, C., Caruana, R., Niculescu-Mizil, A.: Model compression. In: Proceedings of the 12th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, pp. 535–541 (2006)
Culhane, J.F., Goldenberg, R.L.: Racial disparities in preterm birth. In: Seminars in Perinatology, vol. 35, pp. 234–239. Elsevier (2011)
Galimberti, U.: Man in the age of technology. J. Anal. Psychol. 54(1), 3–17 (2009)
Giovanola, B., Tiribelli, S.: Weapons of moral construction? On the value of fairness in algorithmic decision-making. Ethics Inf. Technol. 24(1), 1–13 (2022)
Han, S., et al.: DSD: dense-sparse-dense training for deep neural networks. arXiv preprint arXiv:1607.04381 (2016)
Hinton, G., Vinyals, O., Dean, J., et al.: Distilling the knowledge in a neural network, vol. 2, no. 7. arXiv preprint arXiv:1503.02531 (2015)
Howard, A.G., et al.: MobileNets: efficient convolutional neural networks for mobile vision applications. arXiv preprint arXiv:1704.04861 (2017)
LeCun, Y., Denker, J., Solla, S.: Optimal brain damage. In: Advances in Neural Information Processing Systems, vol. 2 (1989)
Lo, S.Y., Hang, H.M., Chan, S.W., Lin, J.J.: Efficient dense modules of asymmetric convolution for real-time semantic segmentation. In: Proceedings of the ACM Multimedia Asia, pp. 1–6 (2019)
Luby, J.L., Baram, T.Z., Rogers, C.E., Barch, D.M.: Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 43(10), 744–751 (2020)
Ma, N., Zhang, X., Zheng, H.T., Sun, J.: ShuffleNet V2: practical guidelines for efficient CNN architecture design. In: Proceedings of the European Conference on Computer Vision (ECCV), pp. 116–131 (2018)
McCay, K.D., Ho, E.S., Shum, H.P., Fehringer, G., Marcroft, C., Embleton, N.D.: Abnormal infant movements classification with deep learning on pose-based features. IEEE Access 8, 51582–51592 (2020)
Meisels, S.J., Shonkoff, J.P.: Early childhood intervention: a continuing evolution (2000)
Migliorelli, L., Moccia, S., Pietrini, R., Carnielli, V.P., Frontoni, E.: The babypose dataset. Data Brief 33, 106329 (2020)
Moccia, S., Migliorelli, L., Pietrini, R., Frontoni, E.: Preterm infants’ limb-pose estimation from depth images using convolutional neural networks. In: 2019 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), pp. 1–7. IEEE (2019)
Polino, A., Pascanu, R., Alistarh, D.: Model compression via distillation and quantization. arXiv preprint arXiv:1802.05668 (2018)
Prechtl, H.F.: State of the art of a new functional assessment of the young nervous system. An early predictor of cerebral palsy. Early Hum. Dev. 50(1), 1–11 (1997)
Raghuram, K., et al.: Automated movement recognition to predict motor impairment in high-risk infants: a systematic review of diagnostic test accuracy and meta-analysis. Dev. Med. Child Neurol. 63(6), 637–648 (2021)
Reich, S., et al.: Novel AI driven approach to classify infant motor functions. Sci. Rep. 11(1), 1–13 (2021)
Sakkos, D., Mccay, K.D., Marcroft, C., Embleton, N.D., Chattopadhyay, S., Ho, E.S.: Identification of abnormal movements in infants: a deep neural network for body part-based prediction of cerebral palsy. IEEE Access 9, 94281–94292 (2021)
Schmidt, W., Regan, M., Fahey, M., Paplinski, A.: General movement assessment by machine learning: why is it so difficult. J. Med. Artif. Intell. 2 (2019). https://jmai.amegroups.com/article/view/5058. ISSN = 2617-2496
Schwartz, R., Dodge, J., Smith, N.A., Etzioni, O.: Green AI. Commun. ACM 63(12), 54–63 (2020)
Touwen, B.: Variability and stereotypy in normal and deviant development. Clin. Dev. Med. 67, 99–110 (1978)
Viganò, A.: Design and development of a device for the functional evaluation of newborns nervous system in clinical practice (2015)
Wardlaw, T., You, D., Hug, L., Amouzou, A., Newby, H.: UNICEF report: enormous progress in child survival but greater focus on newborns urgently needed. Reprod. Health 11(1), 1–4 (2014)
Yang, H., et al.: Asymmetric 3D convolutional neural networks for action recognition. Pattern Recogn. 85, 1–12 (2019)
Yu, F., Koltun, V.: Multi-scale context aggregation by dilated convolutions. arXiv preprint arXiv:1511.07122 (2015)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Cacciatore, A., Migliorelli, L., Berardini, D., Tiribelli, S., Pigliapoco, S., Moccia, S. (2022). Some Ethical Remarks on Deep Learning-Based Movements Monitoring for Preterm Infants: Green AI or Red AI?. In: Mazzeo, P.L., Frontoni, E., Sclaroff, S., Distante, C. (eds) Image Analysis and Processing. ICIAP 2022 Workshops. ICIAP 2022. Lecture Notes in Computer Science, vol 13374. Springer, Cham. https://doi.org/10.1007/978-3-031-13324-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-13324-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13323-7
Online ISBN: 978-3-031-13324-4
eBook Packages: Computer ScienceComputer Science (R0)