Abstract
Scoring functions are the heart of structure based drug design, where they are used to estimate how strongly the docked pose of a ligand binds to the target. Seeking a scoring function that can accurately predict the binding affinity is key for successful virtual screening methods. Deep learning approaches have recently seen a rise in popularity as a means to improve the scoring function having the key advantage to automatically extract features and create a complex representation of the data without feature engineering and expert knowledge.
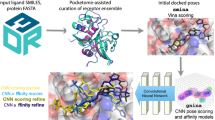
In this study we present the LigityScore scoring functions – LigityScore1D and LigityScore3D. The LigityScore models are rotationally invariant scoring functions based on convolutional neural networks (CNN). LigityScore descriptors are extracted directly from the structural and interacting properties of the protein-ligand complex which are input to a CNN for automatic feature extraction and binding affinity prediction. This representation uses the spatial distribution of Pharmacophoric Interaction Points (PIPs), derived from interaction features from the protein-ligand complex based on pharmacophoric features conformant to specific family types (Hydrogen Bond Acceptor (HBA), Hydrogen Bond Donor (HBD), etc.) and distance thresholds. The data representation component and the CNN architecture together, constitute the LigityScore scoring function.
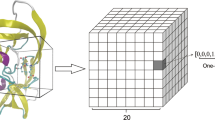
LigityScore1D considers a single distance from each combination of two PIPs from the extracted ligand and protein PIP pools, and generates a feature vector for each pharmacophoric family pair (example HBA-HBD) based on the distribution of the PIP pair distances in discretised space. The different pharmacophoric family pair combinations are grouped to construct a matrix representation of the complex. Similarly, LigityScore3D considers 3-PIP combinations to create a triangular structure with three distances between the PIPs. These are discretised to represent binning coordinates for a 3D feature cube for each pharmacophoric family set (example HBA-HBD-HBD) that describe the distribution of distances in 3D space. The cube from each pharmacophoric family set are grouped together to from a feature cube collection.
The main contribution for this study is to present a novel rotationally invariant protein-ligand representation for use as a CNN based scoring function for binding affinity prediction. The CNN model is used for automatic feature extraction from the LigityScore representations. LigityScore models are evaluated for scoring power on the latest two CASF benchmarks. The Pearson correlation coefficient, and the standard deviation in linear regression were used to compare LigityScore with the benchmark model, and also other models in literature published in recent years. LigityScore3D has achieved better results than LigityScore1D and showed similar performance in both CASF benchmarks. LigityScore3D ranked 5\(^{\textrm{th}}\) place in the CASF-2013 benchmark, and 8\(^{\textrm{th}}\) in CASF-2016, with an average Pearson correlation coefficient (R) performance of 0.713 and 0.725 respectively. LigityScore1D obtained best results when trained using the PBDbind v2018 dataset, and ranked 8\(^{\textrm{th}}\) place in the CASF-2013 and 7\(^{\textrm{th}}\) place in CASF-2016 with an R performance of 0.635 and 0.741 respectively. Our methods show relatively good performance that exceed the Pafnucy performance, as one of the best performing CNN based scoring function, on the CASF-2013 benchmark, using a less computationally complex model that can be trained 16 times faster.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ain, Q.U., Aleksandrova, A., Roessler, F.D., Ballester, P.J.: Machine-learning scoring functions to improve structure-based binding affinity prediction and virtual screening. Wiley Interdisc. Rev.: Comput. Mol. Sci. 5(6), 405–424 (2015)
Azzopardi, J., Ebejer, J.P.: LigityScore: convolutional neural network for binding-affinity predictions. In: Bioinformatics, pp. 38–49 (2021)
Ballester, P.J., Mitchell, J.B.: A machine learning approach to predicting protein-ligand binding affinity with applications to molecular docking. Bioinformatics 26(9), 1169–1175 (2010)
Berman, H., Henrick, K., Nakamura, H.: Announcing the worldwide protein data bank. Nat. Struct. Mol. Biol. 10(12), 980–980 (2003)
Boyles, F., Deane, C.M., Morris, G.M.: Learning from the ligand: using ligand-based features to improve binding affinity prediction. Bioinformatics 36(3), 758–764 (2020)
Ching, T., et al.: Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 15(141), 20170387 (2018)
Dunbar Jr., J.B., et al.: CSAR benchmark exercise of 2010: selection of the protein-ligand complexes. J. Chem. Inf. Model. 51(9), 2036–2046 (2011)
Ebejer, J.P., Finn, P.W., Wong, W.K., Deane, C.M., Morris, G.M.: Ligity: a non-superpositional, knowledge-based approach to virtual screening. J. Chem. Inf. Model. 59(6), 2600–2616 (2019)
Goldberg, Y.: A primer on neural network models for natural language processing. J. Artif. Intell. Res. 57, 345–420 (2016)
Goodfellow, I., Bengio, Y., Courville, A.: Deep Learning. MIT Press, Cambridge (2016)
Gund, P.: Three-dimensional pharmacophoric pattern searching. In: Hahn, F.E., Kersten, H., Kersten, W., Szybalski, W. (eds.) Progress in Molecular and Subcellular Biology, vol. 5, pp. 117–143. Springer, Heidelberg (1977). https://doi.org/10.1007/978-3-642-66626-1_4
He, K., Zhang, X., Ren, S., Sun, J.: Delving deep into rectifiers: surpassing human-level performance on ImageNet classification. In: 2015 IEEE International Conference on Computer Vision (ICCV), pp. 1026–1034 (2015)
Ioffe, S., Szegedy, C.: Batch normalization: accelerating deep network training by reducing internal covariate shift. In: Proceedings of the 32nd International Conference on Machine Learning. Proceedings of Machine Learning Research, vol. 37, pp. 448–456. PMLR, Lille, 07–09 July 2015
Jiménez, J., Skalic, M., Martinez-Rosell, G., De Fabritiis, G.: K deep: protein-ligand absolute binding affinity prediction via 3D-convolutional neural networks. J. Chem. Inf. Model. 58(2), 287–296 (2018)
Kaggle, M.: Kaggle: Merck molecular activity challenge (2012). https://www.kaggle.com/c/MerckActivity, https://www.kaggle.com/c/MerckActivity. Accessed 8 Feb 2019
Kingma, D.P., Ba, J.: Adam: a method for stochastic optimization (2014). arxiv:1412.6980. Comment: Published as a conference paper at the 3rd International Conference for Learning Representations, San Diego, 2015
Landrum, G.: RDKit: open-source cheminformatics (2020). https://www.rdkit.org, accessed April, 2020
Leach, A.R., Gillet, V.J., Lewis, R.A., Taylor, R.: Three-dimensional pharmacophore methods in drug discovery. J. Med. Chem. 53(2), 539–558 (2010)
LeCun, Y., Bengio, Y., Hinton, G.: Deep learning. Nature 521(7553), 436–444 (2015)
Li, Y., et al.: Comparative assessment of scoring functions on an updated benchmark: 1. Compilation of the test set. J. Chem. Inf. Model. 54(6), 1700–1716 (2014)
Li, Y., et al.: Assessing protein-ligand interaction scoring functions with the CASF-2013 benchmark. Nat. Protoc. 13(4), 666 (2018)
Lipinski, C.A., Lombardo, F., Dominy, B.W., Feeney, P.J.: Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23(1–3), 3–25 (1997)
Liu, Z., et al.: Forging the basis for developing protein-ligand interaction scoring functions. Acc. Chem. Res. 50(2), 302–309 (2017)
Liu, Z., Cui, Y., Xiong, Z., Nasiri, A., Zhang, A., Hu, J.: DeepSeqPan, a novel deep convolutional neural network model for pan-specific class i HLA-peptide binding affinity prediction. Sci. Rep. 9(1), 794 (2019)
Lundberg, S.M., Lee, S.I.: A unified approach to interpreting model predictions. In: Advances in Neural Information Processing Systems, vol. 30, pp. 4765–4774. Curran Associates, Inc. (2017)
Ma, J., Sheridan, R.P., Liaw, A., Dahl, G.E., Svetnik, V.: Deep neural nets as a method for quantitative structure-activity relationships. J. Chem. Inf. Model. 55(2), 263–274 (2015)
Mysinger, M.M., Carchia, M., Irwin, J.J., Shoichet, B.K.: Directory of useful decoys, enhanced (dud-e): better ligands and decoys for better benchmarking. J. Med. Chem. 55(14), 6582–6594 (2012)
Nguyen, D.D., Wei, G.W.: AGL-score: algebraic graph learning score for protein-ligand binding scoring, ranking, docking, and screening. J. Chem. Inf. Model. 59(7), 3291–3304 (2019)
Nguyen, D.D., Wei, G.W.: DG-GL: differential geometry-based geometric learning of molecular datasets. International journal for numerical methods in biomedical engineering 35(3), e3179 (2019)
Paszke, A., et al.: PyTorch: an imperative style, high-performance deep learning library. In: Advances in Neural Information Processing Systems, vol. 32, pp. 8024–8035. Curran Associates, Inc. (2019)
Pérez-Sianes, J., Pérez-Sánchez, H., Díaz, F.: Virtual screening meets deep learning. Curr. Comput. Aided Drug Des. 15(1), 6–28 (2019)
Ragoza, M., Hochuli, J., Idrobo, E., Sunseri, J., Koes, D.R.: Protein-ligand scoring with convolutional neural networks. J. Chem. Inf. Model. 57(4), 942–957 (2017)
Rifaioglu, A.S., Atas, H., Martin, M.J., Cetin-Atalay, R., Atalay, V., Dogan, T.: Recent applications of deep learning and machine intelligence on in silico drug discovery: methods, tools and databases. Brief. Bioinform. 10 (2018)
Sieg, J., Flachsenberg, F., Rarey, M.: In need of bias control: evaluating chemical data for machine learning in structure-based virtual screening. J. Chem. Inf. Model. 59(3), 947–961 (2019)
Simonyan, K., Zisserman, A.: Very deep convolutional networks for large-scale image recognition. In: ICLR 2015 (2014)
Stepniewska-Dziubinska, M.M., Zielenkiewicz, P., Siedlecki, P.: Pafnucy-a deep neural network for structure-based drug discovery. Stat 1050, 19 (2017)
Su, M., et al.: Comparative assessment of scoring functions: the CASF-2016 update. J. Chem. Inf. Model. 59(2), 895–913 (2018)
Szegedy, C., et al.: Going deeper with convolutions. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, pp. 1–9 (2015)
Ulyanov, D., Vedaldi, A., Lempitsky, V.: Improved texture networks: maximizing quality and diversity in feed-forward stylization and texture synthesis. In: The IEEE Conference on Computer Vision and Pattern Recognition (CVPR). IEEE (2017)
Wójcikowski, M., Kukiełka, M., Stepniewska-Dziubinska, M.M., Siedlecki, P.: Development of a protein-ligand extended connectivity (PLEC) fingerprint and its application for binding affinity predictions. Bioinformatics 35(8), 1334–1341 (2019)
Zhang, H., Liao, L., Saravanan, K.M., Yin, P., Wei, Y.: DeepBindRG: a deep learning based method for estimating effective protein-ligand affinity. PeerJ 7, e7362 (2019)
Zheng, L., Fan, J., Mu, Y.: OnionNet: a multiple-layer intermolecular-contact-based convolutional neural network for protein-ligand binding affinity prediction. ACS Omega 4(14), 15956–15965 (2019)
Acknowledgements
We would like to thank the AWS Research Credits Team for supporting our research with AWS credits to develop our models.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Azzopardi, J., Ebejer, J.P. (2022). LigityScore: A CNN-Based Method for Binding Affinity Predictions. In: Gehin, C., et al. Biomedical Engineering Systems and Technologies. BIOSTEC 2021. Communications in Computer and Information Science, vol 1710. Springer, Cham. https://doi.org/10.1007/978-3-031-20664-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-20664-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20663-4
Online ISBN: 978-3-031-20664-1
eBook Packages: Computer ScienceComputer Science (R0)