Abstract
Objective: To systematically review the studies related to the functionality and usability evaluation of diabetes mobile apps. Method: We searched three electronic databases: PubMed, Scopus, and Cochrane. The search terms used were “mobile app”, “mobile application”, “diabetes”, and “evaluation”. We limited the articles to those that were written in English and published from January 1, 2006 to October 4, 2016. Results: There were seven articles focused on type 1 diabetes, two articles focused on type 2 diabetes, two articles focused on both type 1 and type 2 diabetes, nine articles focused on diabetes that authors did not state specific type. With regard to types of evaluation, only one study reported solely on functionality, seven studies reported usability, and twelve studies reported both functionality and usability. The methods used for evaluations included survey, interview, laboratory testing, user testing, questionnaire, expert evaluation, and heuristic evaluation. Conclusion: Future studies should consider the standard evaluation methods for evaluate functionality and usability of diabetes self-management (DSM) apps.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Diabetes Self-Management.
Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia [1]. In 2014, there were 29.1 million Americans with diabetes, including 8.1 million people who were undiagnosed [2]. Diabetes self-management education is a process of educating patient the knowledge of diabetes to improve their self-management behaviors [3]. Several studies suggested through appropriate diabetes self-management, diabetic patients can improve the long-term health outcomes [2, 4,5,6,7].
Diabetes Self-Management Applications (Apps).
Mobile health is defined as “mobile computing, medical sensor, and communications technologies” that can improve chronic disease care outside hospitals [8]. In recent years, there has been a rapid development of health apps for smartphones and tablets. Based on the report of IMS Institute for Healthcare Informatics, there were almost 165,000 health apps in 2015 [9]. With this increase in health apps there has also been an increase in the number of apps designed specifically for diabetic patients. In 2013, researchers searched Google Play, App iTunes, and BlackBerry World app stores. They found 1,812 diabetes-related apps [10]. DSM apps are tools on smartphones or tablets designed to help diabetic patients to achieve behavioral changes [11]. DSM apps provide functions, including monitoring carbohydrate intake, exercise, and blood sugar level.
According to the International Standards Organization (ISO 9241-11), usability is defined as “the extent to which a product can be used by specified users to achieve specified goals with effectiveness, efficiency, and satisfaction in a specified context of use” [12]. Usability testing is defined as “a systematic way of observing actual users trying out a product and collecting information about the specific ways in which the product is easy or difficult for them” [13]. Zhang et al. indicated “Usability testing is a mandatory process to ensure that a mobile application is practical, effective, and easy to use, especially from a user’s perspective [14]. The results of less attention to usability may include “frustrated users and decreased efficiency coupled with increased cost [15].
However, limited research and review have been conducted on the functionality and usability evaluation of these apps. The purpose of this review was to systematically review the studies related to the functionality and usability evaluation of diabetes mobile apps.
2 Method
Data Sources.
In October 2016, we searched three electronic databases: PubMed, Scopus, and Cochrane. The search terms used were “mobile app”, “mobile application”, “diabetes”, and “evaluation”. We limited the articles to those that were written in English and published from January 1, 2006 to October 4, 2016.
Inclusion Criteria.
The inclusion criteria were any research related to evaluation on functionality or usability of diabetes applications.
Study Selection and Data Extraction.
We reviewed the titles and abstracts of identified articles. Based on the inclusion criteria, eligible articles were included for full-text review. We collected data from eligible articles, the object of study, diabetes types, study sample, sample size, numbers of apps used in the study, evaluation types (functionality or/and usability), evaluation methods, findings of evaluation, app types (native or web-based app), app platforms (e.g., iOS, Android), device, and evidence-based guidelines used for developing the app.
3 Results
Study Selection.
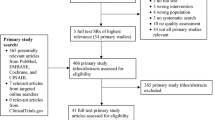
Out of 200 articles, we identified 20 articles as eligible for our systematical literature review (Fig. 1). There were 13 articles from PubMed and 7 articles from Scopus.
Description of Included Studies.
Table 1 shows the summary of functionality and usability studies on diabetes mobile applications. The publication years ranged from 2008 to 2016. There were seven articles focused on type 1 diabetes [16,17,18,19,20,21,22], two articles focused on type 2 diabetes [23, 24], two articles focused on both type 1 and type 2 diabetes [25, 26], and nine articles did not report specific diabetes types [11, 27,28,29,30,31,32,33,34]. Thirteen studies evaluated one in-house mobile app [16, 17, 19,20,21, 23,24,25,26,27,28,29,30] and seven studies evaluated two or more apps developed by others [11, 18, 22, 31,32,33,34]. The number of apps for evaluation ranged from one to 656. Sample size for evaluation ranged from five to 123 subjects. The study sample types included adolescents [16,17,18], adults [19, 20, 24,25,26,27,28, 30], elderly people [23].
Types of Evaluation and Evaluation Methods.
With regard to types of evaluation, only one study reported solely on functionality [21], seven studies reported usability [17, 19, 23, 25, 28,29,30], and twelve studies reported both functionality and usability [11, 16, 18, 20, 22, 24, 26, 27, 31,32,33,34]. The methods used for evaluations included survey [16, 25, 30], interview [17, 18, 24], laboratory testing [17, 20, 21, 27], user testing [18, 19, 22, 23, 25, 26, 28, 32, 34], questionnaire [18,19,20, 23, 26,27,28], expert evaluation [11, 22, 29, 31,32,33], and heuristic evaluation [22, 28, 32, 34].
Apps Types and Platforms.
Out of thirteen studies that evaluated in-house mobile app, two apps were implemented as web-based mobile apps [29, 30]. The other eleven studies did not report app types [16, 17, 19,20,21, 23,24,25,26,27,28].
With regard to the platforms, eleven studies used Android platform [11, 16, 18, 19, 21, 22, 24, 25, 27, 29, 33], six studies used iOS platform [11, 17, 22, 31, 32, 34], two studies used Windows platform [20, 28], one study used Blackberry platform [22], and one study used the Pebble (smartwatch) platform [26]. Two studies did not report the platform types on which the apps developed [23, 30].
Diabetes Guidelines for Developing Apps.
Even though evidence-based guidelines guide effective app development [35], only two studies reported the evidence-based guidelines used when the apps were developed [24, 25]. The other eleven studies which developed apps did not report the guidelines for app development [16, 17, 19,20,21, 23, 26,27,28,29,30].
4 Discussion and Conclusion
This study showed only six studies reported on usability. Usability plays an important role helping users to complete a task successfully with minimal cognitive load [36, 37]. This study revealed that only two studies provided the information about the diabetes guidelines for app development. It is not certain whether the studies used diabetes guidelines but failed to report them, or they did not consider using guideline when developing DSM apps. This result is consistent with the findings from several functionality studies on diabetes apps. Our team had conducted functionality analysis of current diabetes apps to investigate the presence of evidence-based guidelines while developing apps [38]. There were 168 diabetes eligible apps from iOS and Google Play included in the study. The functionality of each app was coded according to the validated AADE7 Self-Care BehaviorsTM by the American Association of Diabetes Educators. The results showed very few apps followed the AADE7 Self-Care BehaviorsTM guideline. Similarly, Chomutare et al., analyzed the functions of 101 DSM apps from Apple iPhone, Google Android, BlackBerry, and Nokia Symbian [35]. The study found that features of diabetes apps on the online market did not follow evidence-based guidelines either [35].
This study showed that most studies used triangular methodologies for functionality and usability evaluation, which was encouraging because mixed methods can reveal more comprehensive usability problems that a single method may not detect [39]. We also found lack of consistency in reporting evaluation findings that some studies did not provide scientific details on study subjects, such as level of prior mobile app experience, health literacy, and education level, which would influence the results evaluation outcomes. For future app development and evaluation, systematic and consistent reporting guideline including the methodical and scientific details should be used to inform research community.
Limitations of the Study.
Our study has limitations. First, we did not consider the clinical outcomes of the diabetes apps because we were interested in evaluation methods. The clinical outcomes may provide additional information on the intervention effect that may be influenced by the functionality or usability. Second, we only included studies that were published in English in our review. Inclusion of the literature published in a language other than English should have expanded the pool of literature.
References
Maniam, A., Dhillon, J.S.: Barriers to the effective use of diabetes self-management applications. In: The 3rd National Graduate Conference (NatGrad 2015). Universiti Tenaga Nasional, Putrajaya Campus (2015)
National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Centers for Disease Control and Prevention (2014)
Norris, S.L., Lau, J., Smith, S.J., Schmid, C.H., Engelgau, M.M.: Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 25(7), 1159–1171 (2002)
Whitlock, L.A., McLaughlin, A.C., Harris, M., Bradshaw, J.: The design of mobile technology to support diabetes self-management in older adults. In: Zhou, J., Salvendy, G. (eds.) DUXU 2015. LNCS, vol. 9194, pp. 211–221. Springer, Cham (2015). doi:10.1007/978-3-319-20913-5_20
Diabetes Prevention Program Research Group: The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 25(12), 2165–2171 (2002)
Suhl, E., Bonsignore, P.: Diabetes self-management education for older adults: general principles and practical application. Diab. Spectr. 19(4), 234–240 (2006)
Lindstrom, J., Louheranta, A., Mannelin, M., Rastas, M., Salminen, V., Eriksson, J., Uusitupa, M., Tuomilehto, J.: The finnish diabetes prevention study (dps): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26(12), 3230–3236 (2003)
Eng, D.S., Lee, J.M.: The promise and peril of mobile health applications for diabetes and endocrinology. Pediatr. Diab. 14(4), 231–238 (2013)
Aitken, M.: Patient Adoption of mHealth Use, Evidence and Remaining Barriers to Mainstream Acceptance (2015)
Martinez-Perez, B., de la Torre-Diez, I., Lopez-Coronado, M.: Mobile health applications for the most prevalent conditions by the World Health Organization: review and analysis. J. Med. Internet Res. 15(6), e120 (2013)
Arnhold, M., Quade, M., Kirch, W.: Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J. Med. Internet Res. 16(4), e104 (2014)
ISO 9241-11: Ergonomic Requirements for Office Work with Visual Display Terminals (VDTs): Part 11: Guidance on Usability. 1 ed. 1998: International Organization for Standardization. 22
Dumas, J.F., Redish, J.C.: A Practical Guide to Usability Testing, p. 412. Greenwood Publishing Group Inc., Westport (1993)
Zhang, D., Adipat, B.: Challenges, Methodologies, and Issues in the Usability Testing of Mobile Applications. Int. J. Hum.-Comput. Interact. 18(3), 293–308 (2005)
Yen, P.Y., Bakken, S.: Review of health information technology usability study methodologies. J. Am. Med. Inform. Assoc. 19(3), 413–422 (2012)
Padman, R., Jaladi, S., Kim, S., Kumar, S., Orbeta, P., Rudolph, K., Tran, T.: An evaluation framework and a pilot study of a mobile platform for diabetes self-management: insights from Pediatric users. Stud. Health Technol. Inform. 192, 333–337 (2013)
Cafazzo, J.A., Casselman, M., Hamming, N., Katzman, D.K., Palmert, M.R.: Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J. Med. Internet Res. 14(3), e70 (2012)
Froisland, D.H., Arsand, E., Skarderud, F.: Improving diabetes care for young people with type 1 diabetes through visual learning on mobile phones: mixed-methods study. J. Med. Internet Res. 14(4), e111 (2012)
Diouri, O., Place, J., Traverso, M., Georgescu, V., Picot, M.C., Renard, E.: Development of a smartphone application to capture carbohydrate, lipid, and protein contents of daily food: need for integration in artificial pancreas for patients with type 1 diabetes? J. Diab. Sci. Technol. 9(6), 1170–1174 (2015)
Preuveneers, D., Berbers, Y.: Mobile phones assisting with health self-care: a diabetes case study. In: MobileHCI 2008 - Proceedings of the 10th International Conference on Human-Computer Interaction with Mobile Devices and Services (2008)
Anthimopoulos, M., Dehais, J., Shevchik, S., Ransford, B.H., Duke, D., Diem, P., Mougiakakou, S.: Computer vision-based carbohydrate estimation for type 1 patients with diabetes using smartphones. J. Diab. Sci. Technol. 9(3), 507–515 (2015)
Garcia, E., Martin, C., Garcia, A., Harrison, R., Flood, D.: Systematic Analysis of Mobile Diabetes Management Applications on Different Platforms. In: Holzinger, A., Simonic, K.-M. (eds.) USAB 2011. LNCS, vol. 7058, pp. 379–396. Springer, Heidelberg (2011). doi:10.1007/978-3-642-25364-5_27
Rollo, M.E., Ash, S., Lyons-Wall, P., Russell, A.: Trial of a mobile phone method for recording dietary intake in adults with type 2 diabetes: evaluation and implications for future applications. J. Telemed. Telecare 17(6), 318–323 (2011)
Waki, K., Aizawa, K., Kato, S., Fujita, H., Lee, H., Kobayashi, H., Ogawa, M., Mouri, K., Kadowaki, T., Ohe, K.: DialBetics with a multimedia food recording tool, FoodLog: smartphone-based self-management for type 2 diabetes. J. Diab. Sci. Technol. 9(3), 534–540 (2015)
Lloyd, B., Groat, D., Cook, C.B., Kaufman, D., Grando, A.: iDECIDE: A mobile application for insulin dosing using an evidence based equation to account for patient preferences. Stud. Health Technol. Inform. 216, 93–97 (2015)
Arsand, E., Muzny, M., Bradway, M., Muzik, J., Hartvigsen, G.: Performance of the first combined smartwatch and smartphone diabetes diary application study. J. Diab. Sci. Technol. 9(3), 556–563 (2015)
Domhardt, M., Tiefengrabner, M., Dinic, R., Fotschl, U., Oostingh, G.J., Stutz, T., Stechemesser, L., Weitgasser, R., Ginzinger, S.W.: Training of carbohydrate estimation for people with diabetes using mobile augmented reality. J. Diab. Sci. Technol. 9(3), 516–524 (2015)
Sultan, S., Mohan, P.: How to interact: evaluating the interface between mobile healthcare systems and the monitoring of blood sugar and blood pressure. In: 2009 6th Annual International Conference on Mobile and Ubiquitous Systems: Networking and Services, MobiQuitous 2009 (2009)
Jabar, M.A., Azmi, M.F., Sidi, F.: Integration of mobile and web application: an implementation of diabetic management system. J. Theor. Appl. Inf. Technol. 55(2), 168–173 (2013)
Garcia-Zapirain, B., de la Torre Diez, I., Sainz de Abajo, B., Lopez-Coronado, M.: Development, technical, and user evaluation of a web mobile application for self-control of diabetes. Telemed. J. E- Health 22(9), 778–785 (2016)
Caburnay, C.A., Graff, K., Harris, J.K., McQueen, A., Smith, M., Fairchild, M., Kreuter, M.W.: Evaluating diabetes mobile applications for health literate designs and functionality. Prev. Chronic Dis. 12, E61 (2015)
Martin, C., Flood, D., Sutton, D., Aldea, A., Harrison, R., Waite, M.: A Systematic Evaluation of Mobile Applications for Diabetes Management. In: Campos, P., Graham, N., Jorge, J., Nunes, N., Palanque, P., Winckler, M. (eds.) INTERACT 2011. LNCS, vol. 6949, pp. 466–469. Springer, Heidelberg (2011). doi:10.1007/978-3-642-23768-3_59
Demidowich, A.P., Lu, K., Tamler, R., Bloomgarden, Z.: An evaluation of diabetes self-management applications for Android smartphones. J. Telemed. Telecare 18(4), 235–238 (2012)
Whitlock, L.A., McLaughlin, A.C: Identifying usability problems of blood glucose tracking apps for older adult users. In: Proceedings of the Human Factors and Ergonomics Society (2012)
Chomutare, T., Fernandez-Luque, L., Arsand, E., Hartvigsen, G.: Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J. Med. Internet Res. 13(3), e65 (2011)
Issa, T., Isaias, P.: Usability and Human Computer Interaction (HCI). In: Issa, T., Isaias, P. (eds.) Sustainable Design, pp. 19–36. Springer, Heidelberg (2015)
Nielsen, J.: Usability 101: Introduction to Usability. (2012). [cited 2017 February 9, 2017]. https://www.nngroup.com/articles/usability-101-introduction-to-usability/
Kim, M.S., Ye, Q., Khan, U., Boren, S.A.: Developing a mobile application to improve diabetic patients’ self-care behaviors: a functionality analysis. AMIA 2016 Annual Symposium (2016)
Lyles, C.R., Sarkar, U., Osborn, C.Y.: Getting a technology-based diabetes intervention ready for prime time: a review of usability testing studies. Curr. Diab. Rep. 14(10), 534 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Ye, Q., Boren, S.A., Khan, U., Kim, M.S. (2017). Evaluation of Functionality and Usability on Diabetes Mobile Applications: A Systematic Literature Review. In: Duffy, V. (eds) Digital Human Modeling. Applications in Health, Safety, Ergonomics, and Risk Management: Health and Safety. DHM 2017. Lecture Notes in Computer Science(), vol 10287. Springer, Cham. https://doi.org/10.1007/978-3-319-58466-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-58466-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58465-2
Online ISBN: 978-3-319-58466-9
eBook Packages: Computer ScienceComputer Science (R0)