Abstract
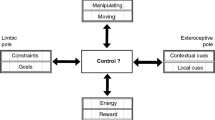
Without a doubt the most sophisticated behavior seen in biological agents is demonstrated by organisms whose behavior is guided by a nervous system. Thus, the construction of behaving devices based on principles of nervous systems may have much to offer. Our group has built series of brain-based devices (BBDs) over the last fifteen years to provide a heuristic for studying brain function by embedding neurobiological principles on a physical platform capable of interacting with the real world. These BBDs have been used to study perception, operant conditioning, episodic and spatial memory, and motor control through the simulation of brain regions such as the visual cortex, the dopaminergic reward system, the hippocampus, and the cerebellum. Following the brain-based model, we argue that an intelligent machine should be constrained by the following design principles: (i) it should incorporate a simulated brain with detailed neuroanatomy and neural dynamics that controls behavior and shapes memory, (ii) it should organize the unlabeled signals it receives from the environment into categories without a priori knowledge or instruction, (iii) it should have a physical instantiation, which allows for active sensing and autonomous movement in the environment, (iv) it should engage in a task that is initially constrained by minimal set of innate behaviors or reflexes, (v) it should have a means to adapt the device’s behavior, called value systems, when an important environmental event occurs, and (vi) it should allow comparisons with experimental data acquired from animal nervous systems. Like the brain, these devices operate according to selectional principles through which they form categorical memory, associate categories with innate value, and adapt to the environment. This approach may provide the groundwork for the development of intelligent machines that follow neurobiological rather than computational principles in their construction.
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Arleo, A., Smeraldi, F., Gerstner, W.: Cognitive navigation based on nonuniform Gabor space sampling, unsupervised growing networks, and reinforcement learning. IEEE Trans. Neural Net. 15, 639–652 (2004)
Aston-Jones, G., Bloom, F.E.: Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosc. 1, 887–900 (1981)
Bienenstock, E.L., Cooper, L.N., Munro, P.W.: Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosc. 2, 32–48 (1982)
Borg-Graham, L.: Modeling the electrical behavior of cortical neurons - simulations of hippocampal pyramidal cells. In: Cotterill, R.M.J. (ed.) Computer Simulation in Brain Science, Cambridge University Press, Cambridge (1987)
Bower, J.M., Beeman, D.: The Book of GENESIS: Exploring Realistic Neural Models with the GEneral NEural SImulation System. TELOS/Springer-Verlag (1994)
Brun, V.H., Otnass, M.K., Molden, S., Steffenach, H.A., Witter, M.P., Moser, M.B., Moser, E.I.: Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 296, 2243–2246 (2002)
Burgess, N., Donnett, J.G., Jeffery, K.J., O’Keefe, J.: Robotic and neuronal simulation of the hippocampus and rat navigation. Philos. Trans. R Soc. Lond. B Biol. Sci. 352, 1535–1543 (1997)
Chiel, H.J., Beer, R.D.: The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 20, 553–557 (1997)
Clark, A.: Being there. Putting brain, body, and world together again. MIT Press, Cambridge (1997)
Doya, K.: Metalearning and neuromodulation. Neural Netw. 15, 495–506 (2002)
Edelman, G.M., Reeke, G.N., Gall, W.E., Tononi, G., Williams, D., Sporns, O.: Synthetic neural modeling applied to a real-world artifact. Proc. Natl. Acad. Sci. USA 89, 7267–7271 (1992)
Edelman, G.M., Reeke Jr., G.N.: Selective networks capable of representative transformations, limited generalizations, and associative memory. Proc. Natl. Acad. Sci. USA 79, 2091–2095 (1982)
Ferbinteanu, J., Shapiro, M.L.: Prospective and retrospective memory coding in the hippocampus. Neuron 40, 1227–1239 (2003)
Fleischer, J.G., Gally, J.A., Edelman, G.M., Krichmar, J.L.: Retrospective and prospective responses arising in a modeled hippocampus during maze navigation by a brain-based device. Proc. Natl. Acad. Sci. USA 104, 3556–3561 (2007)
Friston, K.J., Tononi, G., Reeke, G.N., Sporns, O., Edelman, G.M.: Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience 59, 229–243 (1994)
Geisler, W.S.: Motion streaks provide a spatial code for motion direction. Nature 400, 65–69 (1999)
Guazzelli, A., Bota, M., Arbib, M.A.: Competitive Hebbian learning and the hippocampal place cell system: modeling the interaction of visual and path integration cues. Hippocampus 11, 216–239 (2001)
Hasselmo, M.E., Hay, J., Ilyn, M., Gorchetchnikov, A.: Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw. 15, 689–707 (2002)
Hines, M.L., Carnevale, N.T.: The NEURON simulation environment. Neural Comput. 9, 1179–1209 (1997)
Itti, L.: Automatic foveation for video compression using a neurobiological model of visual attention. IEEE Trans. Image Process 13, 1304–1318 (2004)
Izhikevich, E.M., Gally, J.A., Edelman, G.M.: Spike-timing dynamics of neuronal groups. Cereb Cortex 14, 933–944 (2004)
Krekelberg, B., Dannenberg, S., Hoffmann, K.P., Bremmer, F., Ross, J.: Neural correlates of implied motion. Nature 424, 674–677 (2003)
Krichmar, J.L., Edelman, G.M.: Machine psychology: autonomous behavior, perceptual categorization and conditioning in a brain-based device. Cereb Cortex 12, 818–830 (2002)
Krichmar, J.L., Edelman, G.M.: Brain-based devices for the study of nervous systems and the development of intelligent machines. Artif. Life 11, 63–77 (2005)
Krichmar, J.L., Nitz, D.A., Gally, J.A., Edelman, G.M.: Characterizing functional hippocampal pathways in a brain-based device as it solves a spatial memory task. Proc. Natl. Acad. Sci. USA 102, 2111–2116 (2005a)
Krichmar, J.L., Reeke, G.N.: The Darwin Brain-Based Automata: Synthetic Neural Models and Real-World Devices. In: Reeke, G.N., Poznanski, R.R., Lindsay, K.A., Rosenberg, J.R., Sporns, O. (eds.) Modeling in the Neurosciences: From Biological Systems to Neuromimetic Robotics, pp. 613–638. Taylor & Francis, Boca Raton (2005)
Krichmar, J.L., Seth, A.K., Nitz, D.A., Fleischer, J.G., Edelman, G.M.: Spatial navigation and causal analysis in a brain-based device modeling cortical-hippocampal interactions. Neuroinformatics 3, 197–221 (2005b)
McKinstry, J.L., Edelman, G.M., Krichmar, J.L.: A cerebellar model for predictive motor control tested in a brain-based device. Proc. Natl. Acad. Sci. USA (2006)
Medina, J.F., Carey, M.R., Lisberger, S.G.: The representation of time for motor learning. Neuron 45, 157–167 (2005)
Montague, P.R., Dayan, P., Sejnowski, T.J.: A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci 16, 1936–1947 (1996)
Morris, R.: Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984)
O’Keefe, J., Dostrovsky, J.: The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971)
Pinsky, P.F., Rinzel, J.: Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J. Comput. Neurosci. 1, 39–60 (1994)
Prescott, T.J., Montes Gonzalez, F.M., Gurney, K., Humphries, M.D., Redgrave, P.: A robot model of the basal ganglia: Behavior and intrinsic processing. Neural Netw. 19, 31–61 (2006)
Schultz, W., Dayan, P., Montague, P.R.: A neural substrate of prediction and reward. Science 275, 1593–1599 (1997)
Seth, A.K.: Causal connectivity of evolved neural networks during behavior. Network 16, 35–54 (2005)
Seth, A.K., McKinstry, J.L., Edelman, G.M., Krichmar, J.L.: Active sensing of visual and tactile stimuli by brain-based devices. International Journal of Robotics and Automation 19, 222–238 (2004)
Song, S., Miller, K.D., Abbott, L.F.: Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 3, 919–926 (2000)
Sporns, O., Alexander, W.H.: Neuromodulation and plasticity in an autonomous robot. Neural Netw. 15, 761–774 (2002)
Thierry, A.M., Gioanni, Y., Degenetais, E., Glowinski, J.: Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10, 411–419 (2000)
Wolpert, D., Miall, R., Kawato, M.: Internal models in the cerebellum. Trends in Cognitive Sciences 2, 338–347 (1998)
Worgotter, F., Porr, B.: Temporal sequence learning, prediction, and control: a review of different models and their relation to biological mechanisms. Neural Comput. 17, 245–319 (2005)
Yu, A.J., Dayan, P.: Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005)
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2008 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Krichmar, J.L., Edelman, G.M. (2008). Design Principles and Constraints Underlying the Construction of Brain-Based Devices. In: Ishikawa, M., Doya, K., Miyamoto, H., Yamakawa, T. (eds) Neural Information Processing. ICONIP 2007. Lecture Notes in Computer Science, vol 4985. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-69162-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-540-69162-4_17
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-69159-4
Online ISBN: 978-3-540-69162-4
eBook Packages: Computer ScienceComputer Science (R0)