Abstract
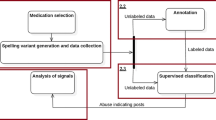
Adverse drug reactions have become one of the top causes of deaths. For surveillance of adverse drug events, patients have gradually become involved in reporting their experiences with medications through the use of dedicated and structured systems. The emerging of social networking provides a way for patients to describe their drug experiences online in less-structured free text format. We developed a computational approach that collects, processes and analyzes Twitter data for drug effects. Our approach uses a machine-learning-based classifier to classify personal experience tweets, and use NLM’s MetaMap software to recognize and extract word phrases that belong to drug effects. Our results on 5 medications demonstrate the validity of our approach, and its ability to correctly extract potential drug effects from the Twitter data. It is conceivable that social media data can serve to complement and/or supplement traditional time-consuming and costly surveillance methods.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
World Health Organization. Pharmacovigilance: ensuring the safe use of medicines. Geneva, Switzerland: World Health Organization (2004)
Dal Pan, G.J.: Monitoring the safety of medicines used off-label. Clin. Pharmacol. Ther. 91(5), 787–795 (2012)
Edwards, B.J., Gounder, M., McKoy, J.M., Boyd, I., Farrugia, M., Migliorati, C., et al.: Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 9(12), 1166–1172 (2008)
Pirmohamed, M., James, S., Meakin, S., Green, C., Scott, A.K., Walley, T.J., et al.: Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 329(7456), 15–19 (2004)
Lazarou, J., Pomeranz, B.H., Corey, P.N.: Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15), 1200–1205 (1998)
Moore, T.J., Cohen, M.R., Furberg, C.D.: Serious adverse drug events reported to the Food and Drug Administration, 1998-2005. Arch. Intern. Med. 167(16), 1752–1759 (2007)
Levinson, D.R., General, I.: Adverse events in hospitals: national incidence among Medicare beneficiaries. Department of Health & Human Services (2010)
Landow, L.: Monitoring adverse drug events: the Food and Drug Administration MedWatch reporting system. Regional Anesthesia and Pain Medicine 23(6), 190–193 (1998)
Edwards, I.R.: Pharmacovigilance. Br. J. Clin. Pharmacol. 73(6), 979–982 (2012)
Egberts, T.C., Smulders, M., de Koning, F.H., Meyboom, R.H., Leufkens, H.: Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ 313(7056), 530–531 (1996)
Chunara, R., Andrews, J.R., Brownstein, J.S.: Social and news media enable estimation of epidemiological patterns early in the, Haitian cholera outbreak. Am J. Trop. Med. Hyg. 86(1), 39–45 (2010)
Stone, B.: https://blog.twitter.com/2009/whats-happening2009 (cited 2013)
Signorini, A., Segre, A.M., Polgreen, P.: The use of Twitter to track levels of disease activity and public concern in the U.S. during the influenza A H1N1 pandemic. PLoS One 6(5), e19467 (2011)
Heaivilin, N., Gerbert, B., Page, J.E., Gibbs, J.L.: Public health surveillance of dental pain via Twitter. J. Dent. Res. 90(9), 1047–1051 (2011)
Corley, C.D., Mikler, A.R., Singh, K.P., Cook, D.J.: Monitoring influenza trends through mining social media. In: International Conference on Bioinformatics & Computational Biology (2009)
Knezevic, M.Z., Bivolarevic, I.C., Peric, T.S., Jankovic, S.M.: Using Facebook to increase spontaneous reporting of adverse drug reactions. Drug Saf. 34(4), 351–352 (2011)
Leaman, R., Wojtulewicz, L., Sullivan, R., Skariah, A., Yang, J., Gonzalez, G.: Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. In: Proceedings of the 2010 Workshop on Biomedical Natural Language Processing. Association for Computational Linguistics (2010)
Verma, S., Vieweg, S., Corvey, W.J., Palen, L., Martin, J.H., Palmer, M., et al.: Natural Language Processing to the Rescue?: Extracting ‘Situational Awareness’ Tweets During Mass Emergency. In: Proc ICWSM (2011)
Elgersma, E., de Rijke, M.: Personal vs non-personal blogs: Initial classification experiments. In: Proceedings of the 31st Annual International ACM SIGIR Conference on Research and Development in Information Retrieval. ACM (2008)
Li, S., Huang, C.-R., Zhou, G., Lee, S.: Employing personal/impersonal views in supervised and semi-supervised sentiment classification. In: Proceedings of the 48th Annual Meeting of the Association for Computational Linguistics. Association for Computational Linguistics (2010)
Bird, S.: NLTK: The natural language toolkit. In: Proceedings of the COLING/ACL on Interactive Presentation Sessions. Association for Computational Linguistics (2006)
Aronson, A.R., Lang, F.M.: An overview of MetaMap: historical perspective and recent advances. J. Am. Med. Inform. Assoc. 17(3), 229–236 (2010)
Bian, J., Topaloglu, U., Yu, F.: Towards large-scale twitter mining for drug-related adverse events. In: Proceedings of the 2012 International Workshop on Smart Health and Wellbeing, pp. 25–32. ACM (2012)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Jiang, K., Zheng, Y. (2013). Mining Twitter Data for Potential Drug Effects. In: Motoda, H., Wu, Z., Cao, L., Zaiane, O., Yao, M., Wang, W. (eds) Advanced Data Mining and Applications. ADMA 2013. Lecture Notes in Computer Science(), vol 8346. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-53914-5_37
Download citation
DOI: https://doi.org/10.1007/978-3-642-53914-5_37
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-53913-8
Online ISBN: 978-3-642-53914-5
eBook Packages: Computer ScienceComputer Science (R0)