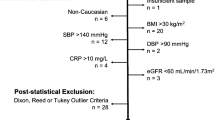
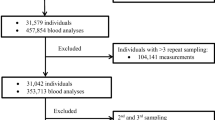
Abstract
Reference intervals (RIs) are fundamental values accompanying medical laboratory results that allow interpretation by medical practitioners, thus influencing patient management. Traditionally, RIs are established by recruiting 120 healthy reference individuals and applying statistical analysis to the results. This method is challenging due to the technical and ethical issues involved. Therefore, many laboratories either adapt RIs provided by the manufacturers of their analytical platforms or the results of RI studies done in other countries. The advent of data mining technology has allowed an alternative method, the indirect RIs (IRIs) approach, which applies appropriate statistical techniques to patient data stored in the laboratory electronic medical records to establish the IRIs. This review briefly highlights the historical aspect of IRI determination, provides a general outline of the steps involved and reviews publications that have used data mining to establish the paediatric IRI over the past ten years.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Coenen, F.: Data mining: past, present and future. Knowl. Eng. Rev. 26(1), 25–29 (2011)
Fayyad, U., Piatetsky-Shapiro, G., Smyth, P.: From data mining to knowledge discovery: an overview. In: Advances in Knowledge Discovery and Data Mining, Editor. AAAI Press/The MIT Press, Menlo Park (1996)
Hoq, M., et al.: Paediatric reference intervals: current status, gaps, challenges and future considerations. Clin. Biochem. Rev. 41(2), 43–52 (2020)
Katayev, A., Balciza, C., Seccombe, D.W.: Establishing reference intervals for clinical laboratory test results: is there a better way? Am. J. Clin. Pathol. 133(2), 180–186 (2010)
Ceriotti, F.: Establishing pediatric reference intervals: a challenging task. Clin. Chem. 58(5), 808–810 (2012)
Mat Nayan, N.: Model for monitoring chronic disease in MHealth applications: a case of Asian country. IRAJ (2020)
Tahmasebi, H., et al.: Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC 28(1), 43–63 (2017)
Lyle, A.N., et al.: Current state of pediatric reference intervals and the importance of correctly describing the biochemistry of child development: a review. JAMA Pediatr. 176(7), 699–714 (2022)
Ibrahim, R., et al.: Estimation of cost of diagnostic laboratory services using activity based costing (ABC) for implementation of Malaysia diagnosis related group (MY-DRG®) in a teaching hospital. Malays. J. Publ. Health Med. 17, 1–8 (2017)
Jones, G.R.D., et al.: Indirect methods for reference interval determination – review and recommendations. Clin. Chem. Lab. Med. (CCLM) 57(1), 20–29 (2019)
Zierk, J., et al.: Indirect determination of hematology reference intervals in adult patients on Beckman Coulter UniCell DxH 800 and Abbott CELL-DYN Sapphire devices. Clin. Chem. Lab. Med. 57(5), 730–739 (2019)
Yang, D., Su, Z., Zhao, M.: Big data and reference intervals. Clin. Chim. Acta 527, 23–32 (2022)
Hoffmann, G., Lichtinghagen, R., Wosniok, W.: Simple estimation of reference intervals from routine laboratory data. Lab. Medizin 39(6) (2016)
Bhattacharya, C.G.: A simple method of resolution of a distribution into gaussian components. Biometrics 23(1), 115–135 (1967)
Horn, P.S., Pesce, A.J., Copeland, B.E.: A robust approach to reference interval estimation and evaluation. Clin. Chem. 44(3), 622–631 (1998)
Beasley, C.M., Jr., et al.: Adaptation of the robust method to large distributions of reference values: program modifications and comparison of alternative computational methods. J. Biopharm. Stat. 29(3), 516–528 (2019)
Grossi, E., et al.: The REALAB project: a new method for the formulation of reference intervals based on current data. Clin. Chem. 51(7), 1232–1240 (2005)
Arzideh, F., et al.: A plea for intra-laboratory reference limits. part 2. a bimodal retrospective concept for determining reference limits from intra-laboratory databases demonstrated by catalytic activity concentrations of enzymes. Clin. Chem. Lab. Med. 45(8), 1043–1057 (2007)
Farrell, C.L., Nguyen, L., Carter, A.C.: Data mining for age-related TSH reference intervals in adulthood. Clin. Chem. Lab. Med. 55(10), e213–e215 (2017)
Lo Sasso, B., et al.: Reference interval by the indirect approach of serum thyrotropin (TSH) in a Mediterranean adult population and the association with age and gender. Clin. Chem. Lab. Med. 57(10), 1587–1594 (2019)
Mrosewski, I., et al.: Indirectly determined hematology reference intervals for pediatric patients in Berlin and Brandenburg. Clin. Chem. Lab. Med. 60(3), 408–432 (2021)
Zierk, J., et al.: Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin. Chem. 61(7), 964–973 (2015)
Zierk, J., Metzler, M., Rauh, M.: Data mining of pediatric reference intervals. J. Lab. Med. 45(6), 311–317 (2021)
Higgins, V., Adeli, K.: Advances in pediatric reference intervals: from discrete to continuous. J. Lab. Prec. Med. 3(1), 77–82 (2018)
Omosule, C.L., et al.: Pediatric ionised calcium reference intervals from archived radiometer data. Clin. Biochem. 104, 13–18 (2022)
Gallo, S., et al.: Redefining normal bone and mineral biochemistry reference intervals for healthy infants in Canada. Clin. Biochem. 47(15), 27–32 (2014)
Gennai, I., et al.: Age- and sex-matched reference curves for serum collagen type I C-telopeptides and bone ALP in children and adolescents: an alternative multivariate statistical analysis approach. Clin. Biochem. 49(10–11), 802–807 (2016)
Monneret, D., et al.: Reference percentiles for paired arterial and venous umbilical cord blood gases: An indirect nonparametric approach. Clin. Biochem. 67, 40–47 (2019)
Ahmed, S., Zierk, J., Khan, A.H.: Establishment of reference intervals for Alkaline phosphatase in Pakistani children using a data mining approach. Lab. Med. 51(5), 484–490 (2020)
Ahmed, S., et al.: Indirect determination of serum creatinine reference intervals in a Pakistani pediatric population using big data analytics. World J. Clin. Pediatr. 10(4), 72–78 (2021)
Ammer, T., et al.: RefineR: a novel algorithm for reference interval estimation from real-world data. Sci. Rep. 11(1), 16023 (2021)
Ha, F., et al.: The reference intervals of whole blood copper, zinc, calcium, magnesium, and iron in infants under 1 year old. Biol. Trace Elem. Res. 200(1), 1–12 (2022)
Dathan-Stumpf, A., et al.: Pediatric reference data of serum lipids and prevalence of dyslipidemia: results from a population-based cohort in Germany. Clin. Biochem. 49(10–11), 740–749 (2016)
Grecu, D.S., et al.: Quality in post-analytical phase: indirect reference intervals for neonates erythrocyte parameters. Clin. Biochem. 46(7–8), 617–621 (2013)
Zierk, J., et al.: Indirect determination of pediatric blood count reference intervals. Clin. Chem. Lab. Med. 51(4), 863–872 (2013)
Weidhofer, C., et al.: Dynamic reference intervals for coagulation parameters from infancy to adolescence. Clin. Chim. Acta 482, 124–135 (2018)
Zierk, J., et al.: Next-generation reference intervals for pediatric hematology. Clin. Chem. Lab. Med. 57(10), 1595–1607 (2019)
Zeljkovic, A., et al.: Indirect reference intervals for haematological parameters in capillary blood of pre-school children. Biochemia Medica 31(1), 134–142 (2021)
Bracho, F.J.: Reference intervals of automated reticulocyte count and immature reticulocyte fraction in a pediatric population. Int. J. Lab. Hematol. 44(3), 461–467 (2022)
Shaw, J.L.V., et al.: Validity of establishing pediatric reference intervals based on hospital patient data: a comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. J. Clin. Biochem. 47(3), 166–172 (2014)
Ozarda, Y., et al.: Comparison of reference intervals derived by direct and indirect methods based on compatible datasets obtained in Turkey. Clinica Chimica Acta: Int. J. Clin. Chem. 520, 186–195 (2021)
Haeckel, R.: Indirect approaches to estimate reference intervals. J. Lab. Med. 45(2), 31–33 (2021)
Chaler, E.A., et al.: Age-specific thyroid hormone and thyrotropin reference intervals for a pediatric and adolescent population. Clin. Chem. Lab. Med. 50(5), 885–890 (2012)
Elmlinger, M.W., et al.: Reference intervals from birth to adulthood for serum thyroxine (T4), triiodothyronine (T3), free T3, free T4, thyroxine binding globulin (TBG) and thyrotropin (TSH). Clin. Chem. Lab. Med. 39(10), 973–979 (2001)
Strich, D., Edri, S., Gillis, D.: Current normal values for TSH and FT3 in children are too low: evidence from over 11,000 samples. J. Pediatr. Endocrinol. Metab. 25(3–4), 245–248 (2012)
Aitkenhead, H., Heales, S.J.: Establishment of paediatric age-related reference intervals for serum prolactin to aid in the diagnosis of neurometabolic conditions affecting dopamine metabolism. Ann. Clin. Biochem. 50(2), 156–158 (2013)
Cook, J., et al.: Pediatric reference ranges for prolactin. Clin. Chem. 38(6), 959 (1992)
Roizen, J.D., et al.: Determination of reference intervals for serum total calcium in the vitamin d-Replete pediatric population. J. Clin. Endocrinol. Metab. 98(12), E1946–E1950 (2013)
Imamoglu, E.Y., et al.: Nomogram-based evaluation of thyroid function in appropriate-for-gestational-age neonates in intensive care unit. J. Perinatol. 35(3), 204–207 (2015)
Søeby, K., et al.: Mining of hospital laboratory information systems: a model study defining age- and gender-specific reference intervals and trajectories for plasma creatinine in a pediatric population. Clin. Chem. Lab. Med. 53(10), 1621–1630 (2015)
Den Elzen, W.P.J., et al.: NUMBER: Standardised reference intervals in the Netherlands using a ‘big data’ approach. Clin. Chem. Lab. Med. 57(1), 42–56 (2018)
Zierk, J., et al.: High-resolution pediatric reference intervals for 15 biochemical analytes described using fractional polynomials. Clin. Chem. Lab. Med. 59(7), 1267–1278 (2021)
ÖZYÜREK, E., et al.: Complete blood parameters for healthy, SGA, full-term newborns. Clin. Lab. Haematol. 28(2), 97–104 (2006)
Grecu, D.S., Paulescu, E.: Quality assurance in the laboratory testing process: indirect estimation of the reference intervals for platelet parameters in neonates. Clin. Biochem. 47(15), 33–37 (2014)
Wasiluk, A., et al.: Platelet indices in SGA newborns. Adv. Med. Sci. 56(2), 361–365 (2011)
Toulon, P., et al.: Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb. Haemost. 116(1), 9–16 (2016)
Zierk, J., et al.: Indirect determination of hematology reference intervals in adult patients on Beckman Coulter UniCell DxH 800 and Abbott CELL-DYN Sapphire devices. Clin. Chem. Lab. Med. (CCLM) 57(5), 730–739 (2019)
Herklotz, R., et al.: Metaanalysis of reference values in hematology. Ther. Umsch. 63(1), 5–24 (2006)
Fleming, J.K., et al.: Development of nation-wide reference intervals using an indirect method and harmonised assays. Clin. Biochem. 99, 20–59 (2022)
Sung, J.Y., et al.: Establishment of pediatric reference intervals for routine laboratory tests in korean population: a retrospective multicenter analysis. Ann. Lab. Med. 41(2), 155 (2021)
Griffiths, J.K., et al.: Centile charts II: alternative nonparametric approach for establishing time-specific reference centiles and assessment of the sample size required. Clin. Chem. 50(5), 907–914 (2004)
Koduah, M., Iles, T.C., Nix, B.J.: Centile charts I: new method of assessment for univariate reference intervals. Clin. Chem. 50(5), 901–906 (2004)
Loh, T.P., et al.: Development of paediatric biochemistry centile charts as a complement to laboratory reference intervals. Pathology 46(4), 336–343 (2014)
Arzideh, F., et al.: An improved indirect approach for determining reference limits from intra-laboratory data bases exemplified by concentrations of electrolytes/Ein verbesserter indirekter Ansatz zur Bestimmung von Referenzgrenzen mittels intra-laboratorieller Datensätze am Beispiel von Elektrolyt-Konzentrationen. J. Lab. Med. 33(2), 52–66 (2009)
Arzideh, F., Wosniok, W., Haeckel, R.: Reference limits of plasma and serum creatinine concentrations from intra-laboratory data bases of several German and Italian medical centres: comparison between direct and indirect procedures. Clin. Chim. Acta 411(3–4), 215–221 (2010)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Nasuruddin, D.N., Salwana, E., Sarker, M.R., Ali, A., Loh, T.P. (2024). Data Mining in Establishing the Indirect Reference Intervals of Biochemical and Haematological Assays in the Paediatric Population: A Review. In: Badioze Zaman, H., et al. Advances in Visual Informatics. IVIC 2023. Lecture Notes in Computer Science, vol 14322. Springer, Singapore. https://doi.org/10.1007/978-981-99-7339-2_41
Download citation
DOI: https://doi.org/10.1007/978-981-99-7339-2_41
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7338-5
Online ISBN: 978-981-99-7339-2
eBook Packages: Computer ScienceComputer Science (R0)