Summary
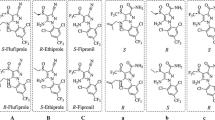
X-ray database searches and theoretical potential-energy calculations indicate that the acid moieties of pyrethroid cyclopropanecarboxylate esters adopt a well-defined, relatively inflexible conformation. In contrast, the alcohol moieties can exist in many low-energy geometries. One of the least conformationally flexible pyrethroid alcohols is 4-phenylindan-2-ol. The approximate overall conformation adopted at the biological binding site by insecticidal esters of this alcohol can be deduced with reasonable confidence by molecular modelling. Graphics superposition of a variety of pyrethroid acids suggests the existence of a large but rather narrow pocket at the binding site, in which substituents at the 3-position of the cyclopropane ring can be accommodated. This pocket is asymmetric with respect to the plane of the cyclopropane ring, extending further on the side remote from the ester group. The effects of α-substitution on the insecticidal activity of pyrethroid esters may be due to the influence of substituents on the preferred conformations of the molecules. This hypothesis rationalises the paradoxical dependence on absolute stereochemistry of the activities of various allylbenzyl and cinnamyl alcohol derivatives.
Similar content being viewed by others
References
Roche M. and Guillet J.C., Pestic. Sci., 16 (1985) 511.
Elliott M. and Janes N.F., Chem. Soc. Rev., 7 (1978) 473.
Byberg J.R., Jorgensen F.S. and Klemmensen P.D., J. Comput-Aided Mol. Design, 1 (1987) 181.
Hopfinger A.J., Malhotra D., Battershell R.D. and Ho A.W., J. Pestic. Sci., 9 (1984) 631.
Allen F.H., Bellard S., Brice M.D., Cartwright B.A., Doubleday A., Higgs H., Hummelink T., Hummelink-Peters B.G., Kennard O., Motherwell W.D.S., Rodgers J.R. and Watson D.G., Acta Crystallogr., B35 (1979) 2331.
Hehre W.J., Radom L., Schleyer P. von R. and Pople J.A., Ab Initio Molecular Orbital Theory, Wiley, New York, NY, 1986, pp. 133–226.
Binkley J.S., Whiteside R.A., Raghavachari K., Seeger R., DeFrees D.J., Schlegel H.B., Frisch M.J., Pople J.A. and Kahn L.R., GAUSSIAN82, Release A, Carnegie-Mellon University, Pittsburgh, PA, 1982.
Guest M.F. and Sherwood P., GAMESS User's Guide, SERC Daresbury Laboratory, Daresbury, 1990.
QCPE Program No. 514, Bloomington, IN, 1986.
Clark M., CramerIII R.D. and Van Opdenbosch N., J. Comput. Chem., 10 (1989) 982.
Tripos Associates, St. Louis, MO, 1992.
Engel, J.F., Staetz, C.A., Young, S.T. and Crosby, G.A., In Janes, N.F. (Ed.) Recent Advances in the Chemistry of Insect Control, Chemical Society Special Publication No. 53, London, 1985, pp. 162–177.
Engel, J.F., U.S. Patent US4335252, 1982.
Hoffmann R., Tetrahedron Lett., 43 (1965) 3819.
Schweizer W.B. and Dunitz J.D., Helv. Chim. Acta, 65 (1982) 1547.
Hata T., Sato S. and Tamura C., Acta Crystallogr., C42 (1986) 449.
Plummer E.L., Seiders R.P., Seelye D.E. and Stewart R.R., Pestic. Sci., 15, (1984) 509.
Engel J.F., Plummer E.L., Stewart R.R., VanSaun W.A., Montgomery R.E., Cruickshank P.A., Harnish W.N., Nethery A.A. and Crosby G.A., In Junshi M. and Kearney P.C. (Eds.) Pesticide Chemistry: Human Welfare and the Environment, Proceedings of the 5th International Congress of Pesticide Chemistry, Vol. 2, Pergamon, Oxford, 1983, pp. 101–106.
Casida J.E., Pyrethrum—The Natural Insecticide, Academic Press, New York, NY, 1973.
Bushell M.J., In Crombie L. (Ed.) Recent Advances in the Chemistry of Insect Control II, Royal Society of Chemistry, Cambridge, 1990, pp. 125–141.
Brown D.G. and Addor R.W., In Geissbuehler H. (Ed.) Advances in Pesticide Science, Plenary Lectures presented at the 4th International Congress of Pesticide Chemistry, Vol. 2, Pergamon, Oxford, 1979, pp. 190–195.
Tsushima K., Matsuo N., Itaya N., Yano T. and Hatakoshi M., In Junshi M. and Kearney P.C. (Eds.) Pesticide Chemistry: Human Welfare and the Environment, Proceedings of the 5th International Congress of Pesticide Chemistry, Vol. 2, Pergamon, Oxford, 1983, pp. 91–94.
Martel J.J., In Junshi M. and Kearney P.C. (Eds.) Pesticide Chemistry: Human Welfare and the Environment, Proceedings of the 5th International Congress of Pesticide Chemistry, Vol. 2, Pergamon, Oxford, 1983, pp. 165–170.
Elliott M., Farnham A.W., Janes N.F., Needham P.H., Pulman D.A. and Stevenson J.H., Nature, 246 (1973) 169.
Van der Heijden S.P.N., Griffith E.A.H., Chandler W.D. and Robertson B.E., Can. J. Chem., 53 (1975) 2084.
Malhotra, S.K. and Ricks, M.J., U.S. Patent US4163787, 1979.
Drendel W.B. and Sundaralingam M., Acta Crystallogr., C41 (1985) 950.
Owen J.D., J. Chem. Soc., Perkin Trans. I, 19 (1975) 1865.
Matsuo N., Yano T. and Ohno N., Agric. Biol. Chem., 49 (1985) 3029.
Author information
Authors and Affiliations
Additional information
Supplementary material available from the authors: Five pages with Cartesian coordinates of hypothesised active conformations of compounds 1, 4, 7, 22–27, 34, 42 and 45.
Zeneca Agrochemicals in the U.K. is part of Zeneca Limited.
Rights and permissions
About this article
Cite this article
Mullaley, A., Taylor, R. Conformational properties of pyrethroids. J Computer-Aided Mol Des 8, 135–152 (1994). https://doi.org/10.1007/BF00119864
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00119864