Summary
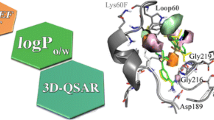
Physical organic structural properties of small molecules and macromolecules such as bond count, branching and proximity between multiple polar fragments contribute significantly to measured hydrophobicity (log P). These structural properties are encoded in the Rekker and Leo methods of calculating log P as structural-dependent factors. Regardless of the size of the atom primitive set, methods predicting log P with only atom primitives can miss subtle structural detail within series of related compounds. The HINT (Hydropathic INTeractions) model for inter- and intramolecular noncovalent interactions calculates atom-based hydrophobic constants, but uses all Leo-type factors in the calculation rather than a large set of atom primitives. Two types of applications of HINT are discussed: evaluation of the binding of an inhibitor (A74704) to HIV-1 protease, where it is shown that modeling of the protonation state (i.e., Asp25, Asp125) in the protein can strongly influence perceived substrate binding; and the use of HINT to calculate a third (hydropathic) field for CoMFA can yield a statistically enhanced and predictive model for molecular design.
Similar content being viewed by others
References
AbrahamD.J. and KelloggG.E., In KubinyiH. (Ed.) 3D QSAR in Drug Design: Theory, Methods and Applications, ESCOM, Leiden, 1993, pp. 506–522.
TanfordC., The Hydrophobic Effect: Formation of Micelles and Biological Membranes, Wiley, New York, NY, 1980, pp. 1–233.
FauchereJ.L. and PliskaV.E., Eur. J. Med. Chem., 18 (1983) 369.
CreightonE., Proteins—Structures and Molecular Properties, Freeman, New York, NY, 1993, pp. 157–163.
HanschC. and LeoA.J., Substituent Constants for Correlation Analysis in Chemistry and Biology, Wiley, New York, NY, 1979, pp. 1–339.
AbrahamD.J. and LeoA.J., Proteins, 2 (1987) 130.
RosemanM.A., J. Mol. Biol., 200 (1988) 513.
EisenbergD., WilcoxW. and McLachlanA.D., J. Cell. Biochem., 31 (1986) 11.
NichollsA., SharpK.A. and HonigB., Proteins, 11 (1991) 281.
FujitaT., IwasaJ. and HanschC., J. Am. Chem. Soc., 86 (1964) 5175.
LeeB. and RichardsF.M., J. Mol. Biol., 55 (1971) 379.
AbrahamD.J., Intra-Science Chem. Rep., 8 (1974) 1.
GhoseA.K. and CrippenG.M., J. Comput. Chem., 7 (1986) 565.
EisenbergD. and McLachlanA.D., Nature, 319 (1986) 199.
AudryE., DubostJ.P., ColleterJ.C. and DalletPh., Eur. J. Med. Chem., 21 (1986) 71.
FuretP., SeleA. and CohenN.C., J. Mol. Graphics, 6 (1988) 182.
FauchereJ.L., QuarendonP. and KaettererL., J. Mol. Graphics, 6 (1988) 203.
WirekoF.C., KelloggG.E. and AbrahamD.J., J. Med. Chem., 34 (1991) 758.
KelloggG.E., JoshiG.S. and AbrahamD.J., Med. Chem. Res., 1 (1992) 444.
KelloggG.E. and AbrahamD.J., J. Mol. Graphics, 10 (1992) 212.
CramerIIIR.D., PattersonD.E. and BunceJ.D., J. Am. Chem. Soc., 110 (1988) 5959.
KelloggG.E., SemusS.F. and AbrahamD.J., J. Comput.-Aided Mol. Design, 5 (1991) 545.
GoodfordP.J., J. Med. Chem., 28 (1985) 849.
FolkersG., MerzA. and RognanD., In WermuthC.G. (Ed.) Trends in QSAR and Molecular Modelling 92 (Proceedings of the 9th European Symposium on Structure-Activity Relationships: QSAR and Molecular Modelling), ESCOM, Leiden, 1993, pp. 233–244.
NorinderU., J. Comput.-Aided Mol. Design, 4 (1990) 381.
PerutzM.F., FermiG., AbrahamD.J., PoyartC. and BursauxE., J. Am. Chem. Soc., 108 (1986) 1064.
Abraham, D.J., (1983) unpublished work.
Abraham, D.J. and Lesk, A., (1985) unpublished work.
Kellogg, G.E. and Abraham, D.J., (1989) unpublished work.
LevittM., J. Mol. Biol., 168 (1983) 595.
LevittM. and PerutzM.F., J. Mol. Biol., 201 (1988) 751.
EricksonJ., NeidhartD.J., VanDrieJ., KempfD.J., WangX.C., NorbeckD.W., PlattnerJ.J., RittenhouseJ.W., TuronM., WideburgN., KohlbrennerW.E., SimmerR., HelfrichR., PaulD.A. and KniggeM., Science, 249 (1990) 527.
Kellogg, G.E., Burt, S., Gussio, R., Erickson, J.E. and Abraham, D.J., manuscript in preparation.
DePriest, S.A., American Crystallographic Association Meeting, Albuquerque, NM, May 23–28, 1993, Abstract G002.
Nayak, V.R. and Kellogg, G.E., Med. Chem. Res., (1994) in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abraham, D.J., Kellogg, G.E. The effect of physical organic properties on hydrophobic fields. J Computer-Aided Mol Des 8, 41–49 (1994). https://doi.org/10.1007/BF00124348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00124348