Summary
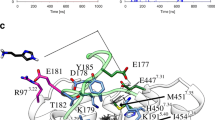
In the first part (pp. 461–478 in this issue) of this study regarding the histamine H2 receptor agonistic binding site, the best possible interactions of histamine with an α-helical oligopeptide, mimicking a part of the fifth transmembrane α-helical domain (TM5) of the histamine H2 receptor, were considered. It was established that histamine can only bind via two H-bonds with a pure α-helical TM5, when the binding site consists of Tyr182/Asp186 and not of the Asp186/Thr190 couple. In this second part, two particular three-dimensional models of G-protein-coupled receptors previously reported in the literature are compared in relation to agonist binding at the histamine H2 receptor. The differences between these two receptor models are discussed in relation to the general benefits and limitations of such receptor models. Also the pros and cons of simplifying receptor models to a relatively easy-to-deal-with oligopeptide for mimicking agonistic binding to an agonistic binding site are addressed. Within complete receptor models, the simultaneous interaction of histamine with both TM3 and TM5 can be analysed. The earlier suggested three-point interaction of histamine with the histamine H2 receptor can be explored. Our results demonstrate that a three-point interaction cannot be established for the Asp98/Asp186/Thr190 binding site in either of the investigated receptor models, whereas histamine can form three H-bonds in case the agonistic binding site is constituted by the Asp98/Tyr182/Asp186 triplet. Furthermore this latter triplet is seen to be able to accommodate a series of substituted histamine analogues with known histamine H2 agonistic activity as well.
Similar content being viewed by others
References
Oliveira L., Paiva A.M.C. and Vriend G., J. Comput.-Aided Mol. Design, 7 (1993) 649.
Vriend, G., ‘Gert Vriend's imagination’, a 3D model of the histamine H2 receptor is available from the TM7 file server.
Timms D., Wilkinson A.J., Kelly D.R., Broadley K.J. and Davies R.H., Recept. Channels, 2 (1994) 107.
Hoflack J., Trumpp-Kallmeyer S. and Hibert M., Trends Pharmacol. Sci., 15 (1994) 7.
Schertler G.F.X., Villa C. and Henderson R., Nature, 362 (1993) 770.
Baldwin J.M., EMBO J., 12 (1993) 1693.
Unger V.M. and Schertler G.F.X., Biophys. J., 68 (1995) 1776.
Gantz I., Munzert G., Tashiro T., Schäffer M., Wang L., DelValle J. and Yamada T., Biochem. Biophys. Res. Commun., 178 (1991) 1386.
Gantz I., DelValle J., Wang L., Tashiro T., Munzert G., Guo Y.-J., Konda Y. and Yamada T., J. Biol. Chem., 267 (1992) 20840.
Nederkoorn P.H.J., Vernooijs P., Donné-Op den Kelder G.M., Baerends E.J. and Timmerman H., J. Mol. Graph., 12 (1994) 242.
Eriks J.C., Van der Goot H. and Timmerman H., Mol. Pharmacol., 44 (1993) 886.
Weinstein H., Mazurek A.P., Osman R. and Topiol S., Mol. Pharmacol., 29 (1986) 28.
Nagy P.I., Durant G.J., Hoss W.P. and Smith D.A., J. Am. Chem. Soc., 116 (1994) 4898.
Sippl W., Stark H. and Höltje H.-D., Quant. Struct.-Act. Relatsh., 14 (1995) 121.
QUANTA/CHARMm, v. 4.0, Simulation, Search, and Analysis, and CHARMm dictionary, Molecular Simulations Inc., Burlington, MA, U.S.A.
Brooks B.R., Bruccoleri R.E., Olafson B.D., States D.J., Swaminathan S. and Karplus M., J. Comput. Chem., 4 (1983) 187.
Chem-X Reference Guide, Chemical Design Ltd., Oxon, U.K., July 1994.
Ippolito J.A., Alexander R.S. and Christianson D.W., J. Biol. Chem., 215 (1990) 457.
Van Duijneveldt-Van de Rijdt J.G.C.M. and Van Duijneveldt F.B., J. Am. Chem. Soc., 93 (1971) 5644.
Smit P.H., Derissen J.L. and Van Duijneveldt F.B., J. Chem. Phys., 67 (1977) 274.
Cambridge Structural Database; Refcode hisahc 10: Bonnet J.J., Jeannin Y. and Laaouini M., Bull. Soc. Fr. Miner. Cri., 98 (1975) 208.
Schlegel H.B., In Lawley K.P. (Ed.) Ab Initio Methods in Quantum Chemistry — I, Wiley, New York, NY, U.S.A., 1987, pp. 249–286.
GAMESS-UK is a package of ab initio programmes written by Guest, M.F., Van Lenthe, J.H., Kendrick, J., Schoeffel, K., Sherwood, P. and Harrison, R.J., with contributions from Amos, R.D., Buenker, R.J., Dupuis, M., Handy, N.C., Hillier, I.H., Knowles, P.J., Bonacic-Koutecky, V., Von Niessen, W., Saunders, V.R. and Stone, A.J. The package is derived from the original GAMESS code, see Ref. 24.
Dupuis, M., Spangler, D. and Wendoloski, J., GAMESS, Natural Resource of Computational Chemistry Software Catalog, Vol. 1, Program No. QG01, 1980.
Guest M.F., Fantucci P., Harrison R.J., Kendrick J., Van Lenthe J.H., Schoeffel K. and Sherwood P., GAMESS-UK User's Guide and Reference Manual, CFS Ltd., Daresbury Laboratory, Daresbury, U.K., 1993.
Némethy G. and Scheraga H.A., Q. Rev. Biophys., 10 (1977) 239.
Darbey N.J. and Creighton T.E. In Rickwood D. (Ed.) Protein Structure, IRL Press, Oxford, U.K., 1993, pp. 1–22.
Ganellin C.R., In Ganellin C.R. and Parsons M.E. (Eds.) Pharmacology of Histamine Receptors, Wright, Bristol, U.K., 1982, pp. 10–102.
Hol W.G.J., Prog. Biophys. Mol. Biol., 45 (1985) 149.
Ter Laak A.M., Leurs R., Smit M.J., Nederkoorn P.H.J., Timmerman H. and Donné-Op den Kelder G.M., J. Comput.-Aided Mol. Design, 9 (1995) 319.
Leurs R., Smith M.J., Meeder R., Ter Laak A.M. and Timmerman H., Biochem. Biophys. Res. Commun. 214 (1995) 110.
Evans S.V., J. Mol. Graph., 11 (1993) 134.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nederkoorn, P.H.J., van Gelder, E.M., Donné-Op den Kelder, G.M. et al. The agonistic binding site at the histamine H2 receptor. II. Theoretical investigations of histamine binding to receptor models of the seven α-helical transmembrane domain. J Computer-Aided Mol Des 10, 479–489 (1996). https://doi.org/10.1007/BF00124477
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00124477