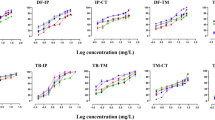
Summary
Quantitative structure-activity relationships (QSARs) for 16 azoxy compounds with antifungal activity have been studied by the combined approach of a partial least-squares method and factorial design. The PLS model equation suggested the structural requirements of two substituents, R1 and R2, for the antifungal activity. The sterically bulky and hydrophobic R1 substituents and electron-withdrawing R2 substituents are favorable for the activity. We propose candidate compounds which are more potent than the compounds based on QSAR data. In this study, we show that the chemometric approach is a powerful tool for QSAR studies and drug design.
Similar content being viewed by others
Abbreviations
- PLS:
-
partial least squares
- FD:
-
factorial design
- MLR:
-
multiple linear regression
- PPs:
-
principal properties
References
Hansch, C. and Fujita, T., J. Am. Chem. Soc., 86 (1964) 1616.
Dunn III, W.J., Chemometrics Intelligent Lab. Syst., 6 (1989) 181.
Kowalski, B.R., Chemometrics; Theory and Application, American Chemical Society, Washington, DC, 1977.
Massart, D.L., Vandeginste, B.G.M., Deming, S.N., Michotte, Y. and Kaufman, L., Chemometrics: A Textbook, Elsevier, Amsterdam, 1988.
Miyashita, Y., Li, Z. and Sasaki, S., Trends Anal. Chem., 12 (1993) 50.
Martens, H. and Næs, T., Multivariate Calibration, Wiley, Chichester, 1989.
Dunn III, W.J., Wold, S., Edlund, U., Hellberg, S. and Gasteiger, J., Quant. Struct.-Act. Relatsh., 3 (1984) 131.
Miyashita, Y., Ohsako, H., Takayama, C. and Sasaki, S., Quant. Struct.-Act. Relatsh., 11 (1992) 17.
Hasegawa, K., Miyashita, Y., Sasaki, S., Sonoki, H. and Shigyou, H., Chemometrics Intelligent Lab. Syst., 16 (1992) 69.
Hellberg, S., Sjöström, M., Skagerberg, B. and Wold, S., J. Med. Chem., 30 (1987) 1126.
Skagerberg, B., Bonelli, D., Clementi, S., Cruciani, G. and Ebert, C., Quant. Struct.-Act. Relatsh., 8 (1989) 32.
De Meo, G., Pedini, M. and Ricci, A., Il Farmaco, 45 (1990) 313.
Box, G.E.P., Hunter, W.G. and Hunter, J.S., Statistics for Experiments, Wiley, New York, NY, 1978.
Deushi, T. et al., manuscript in preparation.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology, Wiley, New York, NY, 1979.
Verloop, A., Hoogenstraaten, W. and Tipker, J., In Äriense, E.J. (Ed.) Drug Design, Vol. 7, Academic Press, New York, NY, 1976, pp. 165–207.
Wold, S., Albano, C., Dunn III, W.J., Esbensen, K., Hellberg, S., Johansson, E. and Sjöström, M., In Martens, H. and Russwurm Jr., H. (Eds.) Food Research and Data Analysis, Applied Science Publishers, London, 1983, pp. 147–188.
Geladi, P. and Kowalski, B.R., Anal. Chim. Acta, 185 (1986) 19.
Höskludsson, A., J. Chemometrics, 2 (1988) 221.
Glen, W.G., Dunn III, W.J. and Scott, D.R., Tetrahedron Comput. Methodol., 2 (1989) 349.
Wold, S., Technometrics, 20 (1978) 397.
Okuyama, T., Miyashita, Y., Kanaya, S., Katsumi, H., Sasaki, S. and Randié, M., J. Comput. Chem., 9 (1988) 636.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasegawa, K., Deushi, T., Yoshida, H. et al. Chemometric QSAR studies of antifungal azoxy compounds. J Computer-Aided Mol Des 8, 449–456 (1994). https://doi.org/10.1007/BF00125379
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00125379