Summary
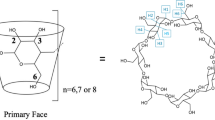
A series of β-cyclodextrin complexes containing various guest molecules was studied using computer-aided molecular modeling and conformation analysis techniques. The geometry of each complex was studied using crystallographic data. The positions of the glycosidic O4 atoms indicate that the β-cyclodextrin molecules are elliptically distorted. This distortion can be related to the van der Waals volume of the guest molecules. This correlation is different for aromatic and non-aromatic guest compounds. Rigid body docking experiments demonstrated that in crystal structures the guest molecule occupies a position in the cavity of nearly minimum interaction energy when there are no other molecules having interactions with the guest molecule. From the crystallographic data several rules could be deduced which seem to determine the conformation of β-cyclodextrin molecules in complexes. A procedure was developed to construct β-cyclodextrin molecules that are able to encompass guest molecules having a given van der Waals volume.
Similar content being viewed by others
References
Uekama, K. and Otagiri, M., CRC Crit. Rev. Ther. Drug Carrier Syst., 3 (1987) 1.
Pagington, J.S., Chem. Br., 23 (1987) 455.
Saenger, W., Angew. Chem. Int. Ed. Engl., 19 (1980) 344.
CSD, Cambridge Crystallographic Data Centre, Cambridge, U.K.
Allen, F.H., Kennard, O. and Taylor, R., Acc. Chem. Res., 16 (1983) 144.
Chem-X, a molecular modelling package, April 89 release, developed and distributed by Chemical Design Ltd, Oxford, U.K.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology, John Wiley & Sons, Inc., New York, NY, 1979.
Leo, A., Hansch, C. and Elkins, D., Chem. Rev., 71 (1971) 525.
Still, W.C. et al., MacroModel version 1.5, Department of Chemistry, Columbia University, New York, NY 10027, U.S.A.
Van Helden, S., van Drooge, M.J., Claessens, A.J., Jansen, A.C.A. and Janssen, L.H.M., Carbohydr. Res., accepted.
Menger, F.M. and Sherrod, M.J., J. Am. Chem. Soc., 110 (1988) 8606.
Arnold, E.N., Lillie, T.S. and Beesley, T.E., J. Liq. Chromatogr., 12 (1989) 337.
Lü, T., Zhang, D. and Dong, S., J. Chem. Soc., 85 (1989) 1439.
Hamilton, J.A., Carbohydr. Res., 142 (1985) 21.
Betzel, C., Hingerty, B., Noltemeyer, M., Weber, G., Saenger, W. and Hamilton, J.A., J. Inclusion Phenom., 1 (1983) 181.
Hamilton, J.A. and Sabesan, M.N., Carbohydr. Res., 102 (1982) 31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kostense, A.S., van Helden, S.P. & Janssen, L.H.M. Modeling and conformation analysis of β-cyclodextrin complexes. J Computer-Aided Mol Des 5, 525–543 (1991). https://doi.org/10.1007/BF00135312
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00135312