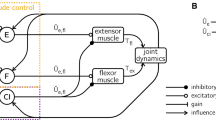
Abstract
In inactive stick insects, sensory information from the femoral chordotonal organ (fCO) about position and movement of the femur-tibia joint is transferred via local nonspiking interneurons onto extensor and flexor tibiae motoneurons. Information is processed by the interaction of antagonistic parallel pathways at two levels: (1) at the input side of the nonspiking interneurons and (2) at the input side of the motoneurons. We tested by a combination of physiological experiments and computer simulation whether the known network topology and the properties of its elements are sufficient to explain the generation of the motor output in response to passive joint movements, that is resistance reflexes. In reinvestigating the quantitative characteristics of interneuronal pathways we identified 10 distinct types of nonspiking interneurons. Synaptic inputs from fCO afferents onto these interneurons are direct excitatory and indirect inhibitory. These connections were investigated with respect to position and velocity signals from the fCO. The results were introduced in the network simulation. The motor output of the simulation has the same characteristics as the real system, even when particular types of interneurons were removed in the simulation and the real system.
Similar content being viewed by others
References
Anastasio TJ, Robinson DA (1990) Distributed parallel processing in the vertical vestibulo-ocular reflex: Learning networks compared to tensor theory. Biol. Cybern. 63:161–167.
Bässler U (1976) Reversal of a reflex to a single motoneuron in the stick insect Carausius morosus. Biol. Cybern. 24:47–49.
Bässler U (1983) Neural basis of elementary behavior in stick insects. Springer Verlag, Berlin.
Bässler U (1986) Afferent control of walking movements in the stick insect Cuniculina impigra. II. Reflex reversal and the release of swing phase in the restrained foreleg. J. Comp. Physiol. A158:351–362.
Bässler U (1988) Functional principles of pattern generation for walking movements of stick insects forelegs: The role of the femoral chordotonal afferences. J. Exp. Biol. 136:125–147.
Bässler U (1993) The femur-tibia control system of stick insects: A model system for the study of the neural basis of joint control. Brain Research Reviews 18:207–226.
Bässler U, Storrer J, Saxer K (1982) The neural basis of catalepsy in the stick insect Cuniculina impigra. 2. The role of the extensor motor neuron and the characteristic of the extensor tibiae muscle. Biol. Cybern. 46:1–6.
Bässler U, Hofmann T, Schuch U (1986) Assisting components within a resistance reflex of the stick insect, Cuniculina impigra. Physiol. Entomol. 11:359–366.
Bässler U, Büschges A (1990) Interneurones participating in the “active reaction” in stick insects Biol. Cybern. 62:529–538.
Bässler U, Nothof U (1994) Gain control in a proprioceptive feedback loop as a prerequisite for working close to instability. J. Comp. Physiol. A174:23–33.
Bergdoll S, Koch UT (1995) BIOSIM: A biological neural network simulator for research and teaching, featuring interactive graphical user interface and learning capabilities. Neurocomputing 8:93–112.
Büschges A (1990) Nonspiking pathways in a joint-control loop of the stick insect Carausius morosus. J. Exp. Biol. 151:133–160.
Büschges A (1994) The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J. Exp. Biol. 189:285–292.
Büschges A, Schmitz J (1991) Nonspiking pathways antagonize the resistance reflex in the thoraco-coxal joint of stick insects. J. Neurobiol. 22:224–237.
Büschges A, Kittmann R, Schmitz J (1994) Identified nonspiking interneurons in leg reflexes and during walking in the stick insect. J. Comp. Physiol. A 174:685–700.
Büschges A, Wolf H (1995) Nonspiking local interneurons in insect leg motor control. I. Common layout and species-specific response properties of femur-tibia joint control pathways in stick insect and locust. J. Neurophysiol. 73:1843–1860.
Burrows M (1987a) Parallel processing of proprioceptive signals by spiking local interneurons and motor neurones in the locust. J. Neurosci. 7:1064–1080.
Burrows M (1987b) Inhibitory interactions between spiking and nonspiking local interneurons in the locust. J. Neurosci. 7: 3282–3292.
Burrows M (1989) Processing of mechanosensory signals in local reflex pathways of the locust. J. Exp. Biol. 146:209–227.
Burrows M (1992) Local circuits for the control of leg movement in an insect. TINS 15:226–232.
Burrows M, Laurent GJ, Field LH (1988) Proprioceptive inputs to nonspiking local interneurons contribute to local reflexes of a locust hindleg. J. Neurosci. 8:3085–3093.
Burrows M, Laurent GJ (1989) Reflex circuits and the control of movement. In: R Durbin, C Miall, G Mitchison, eds. The Computing Neuron. Addison-Wesley Publishing Co., Wokingham. pp. 244–261.
Burrows M, Laurent GJ (1993) Synaptic potentials in the central terminals of locust proprioceptive afferents. J. Neurosci. 13:808–819.
Burrows M, Matheson T (1994) A presynaptic gain control mechanism among sensory neurons of a locust leg proprioceptor. J. Neurosci. 14:272–282.
Cattaert D, El Manira A, Clarac F (1992) Direct evidence for presynaptic inhibitory mechanisms in crayfish sensory afferents. J. Neurophysiol. 67:610–624.
Driesang RB, Büschges A (1993) The neural basis of catalepsy in the stick insect. IV. Properties of nonspiking interneurons. J. Comp. Physiol. A 173:445–454.
Driesang RB, Büschges A (1996) Physiological changes in central neuronal pathways contributing to the generation of a reflex reversal. J. Comp. Physiol. A, in press.
Duysens J, Trippel M, Horstmann GA, Dietz V (1990) Gating and reflex reversal of reflexes in ankle muscles during human walking. Exp. Brain. Res. 82:351–358.
Ebner I, Bässler U (1978) Zur Regelung der Stellung des Femur — Tibia Gelenks im Mesothorax der Wanderheuschrecke Schistocerca gregaria (Forskal). Biol. Cybern. 29:83–96.
Egelhaaf M, Borst A (1993) Motion computation and visual orientation in flies. Comp. Biochem. Physiol. 104A:659–673.
El Manira A, Cattaert D, Clarac F (1991) Monosynaptic connections mediate resistance reflex in crayfish (Procambarus clarkii) walking legs. J. Comp. Physiol. A168:337–349.
Frost WN, Kandel ER (1995) Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J. Neurophysiol. 73:2413–2427.
Grimm K, Sauer AE (1995) The high number of neurons contributes to the robustness of the locust flight-CPG against parameter variation. Biol. Cybern. 72:329–335.
Harris-Warrick RM, Nagy F, Nusbaum MP (1992a) Neuromodulation of stomatogastric networks by identified neurons and transmitters. In: RM Harris-Warrick, E Marder, AI Selverston, M Moulins, eds. Dynamic Biological Networks: The Stomatogastric Nervous System, MIT Press, Boston, pp. 87–137.
Harris-Warrick RM, Marder E, Selverston AI, Moulins M, eds. (1992b) Dynamic Biological Networks. The Stomatogastric Nervous System. MIT Press, Cambridge, Mass.
Hofmann T, Koch UT (1985) Acceleration receptors in the femoral chordotonal organ of the stick insect, Cuniculina impigra. J. Exp. Biol. 114:225–237.
Hofmann T, Koch UT, Bässler U (1985) Physiology of the femoral chordotonal organ in the stick insect, Cuniculina impigra. J. Exp. Biol. 114:207–223.
Kittmann R (1991) Gain control in the femur-tibia feedback system of the stick insect. J. Exp. Biol. 157:503–522.
Laurent G (1990) Voltage-dependent nonlinearities in the membrane of locust nonspiking local interneurons, and their significance for synaptic integration. J. Neurosci. 10:2268–2280.
Laurent G (1991) Evidence for voltage-activated outward currents in the neuropilar membrane of locust nonspiking interneurons. J. Neurosci. 11:1713–1726.
Laurent G, Seymour-Laurent KJ, Johnson K (1993) Dendritic excitability and a voltage-gated calcium current in locust nonspiking local interneurons. J. Neurophysiol. 69:1484–1498.
Lockery SR, Kristan WB (1990a) Distributed processing of sensory information in the leech. I. Input-output relations of the local bending reflex. J. Neurosci. 10:1811–1815.
Lockery SR, Kristan WB (1990b) Distributed processing of sensory information in the leech. II. Identification of interneurons contributing to the local bending reflex. J. Neurosci. 10:1816–1829.
Matheson T (1992) Range fractionation in the locust metathoracic femoral chordotonal organ. J. Comp. Physiol. A170:509–520.
McClelland JL, Rumelhart DE (1988) Parallel Distributed Processing. MIT Press, Cambridge, Mass.
Morton D, Chiel H (1994) Neural architectures for adaptive behavior. TINS 17:413–420.
Nagayama T, Hisada T (1987) Opposing parallel connections through crayfish local nonspiking interneurons. J. Comp. Neurol. 257:347–358.
Namba H, Nagayama T, Hisada M (1994) Descending control of nonspiking local interneurons in the terminal abdominal ganglion of the crayfish. J. Neurophysiol. 72:235–247.
Osborn CE, Popelle RE (1992) Parallel distributed network characteristics of the DSCT. J. Neurophysiol. 68:1100–1112.
Pearson KG (1993) Common principles of motor control in vertebrates and invertebrates. Ann. Rev. Neurosci. 16:265–297.
Pearson KG (1995) Reflex reversal in the walking systems of mammals and arthropods. In: WR Ferrell, U Proske, eds. Neural Control of Movement. Plenum Press, New York. pp. 135–141.
Pearson KG, Wong RKS, Fourtner CR (1976) Connexions between hair-plate afferents and motoneurons in the cockroach leg. J. exp. Biol. 64:251–266.
Pearson KG, Ramirez JM (1992) Parallels with other invertebrate and vertebrate motor systems. In: RM Harris-Warrick, E Marder, AI Selverston, M Moulins, eds. Dynamic Biological Networks: The Stomatogastric Nervous System. MIT Press, Cambridge, MA.
Prochazka A (1989) Sensorimotor gain control: A basic strategy of motor systems? Prog. Neurobiol. 33:281–307.
Ritzmann RE, Pollack AJ (1990) Parallel motor pathways from thoracic interneurons of the ventral giant interneuron system of the cockroach Periplaneta americana. J. Neurobiol. 21:1219–1235.
Rudomin P (1990) Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trend Neurosci. 13:499–505.
Sauer AE, Büschges A (1994) Presynaptic inhibition of afferents — A mechanism influencing gain in proprioceptive feedback systems. In: N Elsner, H Breer, eds. Götingen Neurobiology Report 1994. Georg Thieme Verlag Stuttgart, New York. p. 283.
Sauer AE, Driesang RB, Büschges A, Bässler U (1995) Information processing in the femur-tibia control loop of stick insects 1. The response characteristics of two nonspiking interneurons result from parallel excitatory and inhibitory inputs. J. Comp. Physiol. A177:145–158.
Schmitz J, Delcomyn F, Büschges A (1991) Oil and hook electrodes for en passant recording from small nerves. In: PM Conn, ed. Methods in Neuroscience 4. Academic Press, San Diego, pp. 266–278.
Shepherd GM (1988) Neurobiology. 2nd ed. Oxford University Press, New York.
Skorupski P, Rawat BM, Bush BMH (1992) Heterogenity and central modulation of feedback reflexes in crayfish motor pool. J. Neurophysiol. 67:648–663.
Skorupski P, Sillar KT (1986) Phase-dependent reversal of reflexes mediated by the thoracocoxal muscle receptor organ in the crayfish, Pacifastacus leniusculus. J. Neurophysiol. 55:689–695.
Tsau Y, Wu J-Y, Hopp H-P, Cohen LB, Schiminovich D, Falk CX (1994) Distributed aspects of the response to siphon touch in Aplysia: spread of stimulus information and cross-correlation analysis. J. Neurosci. 14:4167–4184.
Weiland G, Koch UT (1987) Sensory feedback during active movements of stick insects. J. Exp. Biol. 133:137–156.
Wendel O (1993) MOBIS: Ein wissensbasiertes Experimentiersystem zur Simulation biologisch orientierter neuronaler Netze. In: R Hofestädt, F Krückerberg, T Lengauer, eds. Informatik in den Biowissenschaften. Springer-Verlag, Berlin, pp. 203–213.
Wolf H (1991) Sensory feedback in the locust flight patterning. In: DM Armstrong, BMH Busch, eds. Local Neuronal Mechanism in Arthropods and Vertebrates. Manchester University Press, Manchester, pp. 134–148.
Wolf H, Büschges A (1995) Nonspiking local interneurons in insect local motor control. 2. The role of nonspiking local interneurons in the control of leg swing during walking. J. Neurophyiol 73:1861–1875.
Wu J-Y, Cohen LB, Falk CX (1994) Neuronal activity during different behaviors in Aplysia: A distributed organization? Science 263:820–823.
Zecevic D, Wu J-Y, Cohen LB, London JA, Höpp H-P, Falk CX (1989) Hundreds of neurons in the Aplysia abdominal ganglion are active during the gill-withdrawal reflex. J. Neurosc. 9:3681–3689.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sauer, A.E., Driesang, R.B., Büschges, A. et al. Distributed processing on the basis of parallel and antagonistic pathways simulation of the femur-tibia control system in the stick insect. J Comput Neurosci 3, 179–198 (1996). https://doi.org/10.1007/BF00161131
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00161131