Abstract
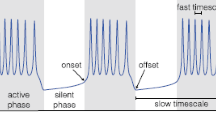
An 11-variable Hodgkin-Huxley type model of a bursting neuron was investigated using numerical bifurcation analysis and computer simulations. The results were applied to develop a reduced model of the underlying subthreshold oscillations (slow-wave) in membrane potential. Two different low-order models were developed: one 3-variable model, which mimicked the slow-wave of the full model in the absence of action potentials and a second 4-variable model, which included expressions accounting for the perturbational effects of action potentials on the slow-wave. The 4-variable model predicted more accurately the activity mode (bursting, beating, or silence) in response to application of extrinsic stimulus current or modulatory agents. The 4-variable model also possessed a phase-response curve that was very similar to that of the original 11-variable model. The results suggest that low-order models of bursting cells that do not consider the effects of action potentials may erroneously predict modes of activity and transient responses of the full model on which the reductions are based. These results also show that it is possible to develop low-order models that retain many of the characteristics of the activity of the higher-order system.
Similar content being viewed by others
References
Alevizos A, Skelton M, Weiss KR, Koester J (1991) A comparison of bursting neurons in Aplysia. Biological Bulletin 180:269–275.
Baer SM, Rinzel J, Carrillo H (1995) Analysis of an autonomous phase model for neuronal parabolic bursting. Journal of Mathematical Biology 33:309–333.
Bertram R (1993) A computational study of the effects of serotonin on a molluscan burster neuron. Biological Cybernetics 69:257–267.
Bertram R, Butte MJ, Kiemel T, Sherman A (1995) Topological and phenomenological classification of bursting oscillations. Bulletin of Mathematical Biology 57:413–439.
Butera RJ, Clark JW, Canavier CC, Baxter DA, Byrne JH (1995) Analysis of the effects of modulatory agents on a modeled bursting neuron: Dynamic interactions between voltage and calcium dependent systems. Journal of Computational Neuroscience 2:19–44.
Canavier CC, Baxter DA, Clark JW, Byrne JH (1993) Nonlinear dynamics in a model neuron provide a novel mechanism for transient synaptic inputs to produce long-term alterations of post-synaptic activity. Journal of Neurophysiology 69:2252–2257.
Canavier CC, Clark JW, Byrne JH (1991) Simulation of the bursting activity of neuron R15 in Aplysia: Role of ionic currents, calcium balance, and modulatory transmitters. Journal of Neurophysiology 66:2107–2124.
Chay TR (1990) Bursting excitable cell models by a slow Ca2+ current. Journal of Theoretical Biology 142:305–315.
Chay TR, Keizer J (1983) Minimal model for membrane oscillations in the pancreatic β-cell. Biophysical Journal 42:181–190.
Doedel EJ (1981) AUTO: A program for the automatic bifurcation and analysis of autonomous systems. Congressus Numerantium 30:265–284.
Dvořák I, Šiška J (1989) Analysis of metabolic systems with complex slow and fast dynamics. Bulletin of Mathematical Biology 51:255–274.
Ermentrout GB (1994) Reduction of conductance-based models with slow synapses to neural nets. Neural Computation 6:679–695.
Hairer E, Wanner G (1990) Solving Ordinary Differential Equations II. Stiff and Differential-algebraic Problems. Springer Series in Computational Mathematics. Springer-Verlag, New York.
Hale J, Koçak H (1991) Dynamics and Bifurcations. Vol. 3 of Texts in Applied Mathematics. Springer-Verlag, New York.
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction excitation in nerve. Journal of Physiology (London) 117:500–544.
Junge D, Stephens CL (1973) Cyclic variation of potassium conductance in a burst generating neurone in Aplysia. Journal of Physiology (London) 235:155–181.
Keizer J, Mangus G (1989) ATP-sensitive potassium channel and bursting in the pancreatic beta-cell: A theoretical study. Biophysical Journal 56:229–242.
Kepler TB, Abbott LF, Marder E (1992) Reduction of conductancebased neuron models. Biological Cybernetics 66:381–387.
Kononenko NI (1994) Dissection of a model for membrane potential oscillations in bursting neuron of snail, Helix pomata. Comparative Biochemistry and Physiology, Part A 107A:323–332.
LoFaro T, Kopell N, Marder E, Hooper SL (1994) Subharmonic coordination in networks of neurons with slow conductances. Neural Computation 6:69–84.
Mathieu PA, Roberge FA (1971) Characteristics of pacemaker oscillations in Aplysia neurons. Canadian Journal of Pharmacology and Physiology 49:787–795.
Morris C, Lecar H (1981) Voltage oscillations in the barnacle muscle fiber. Biophysical Journal 35:193–213.
Pavlidis T (1973) Biological Oscillators: Their Mathematical Analysis. Academic Press, New York.
Pinsker HM (1977) Aplysia bursting neurons as endogenous oscillators. I. Phase-response curves for pulsed inhibitory synaptic input. Journal of Neurophysiology 40:527–543.
Plant RE, Kim M (1975) On the mechanism underlying bursting in the Aplysia abdominal ganglion R15 cell. Mathematical Biosciences 26::357–375.
Plant RE, Kim M (1976) Mathematical description of a bursting pacemaker neuron by a modification of the Hodgkin-Huxley equations. Biophysical Journal 16:227–244.
Rheinboldt W, Burkardt J (1983a) Algorithm 596: A program for a locally parameterized continuation process. ACM Transactions on Mathematical Software 9:236–241.
Rheinboldt W, Burkardt J (1983b) A locally parameterized continuation process. ACM Transactions on Mathematical Software 9:215–235.
Rinzel J (1985) Bursting oscillations in an excitable membrane model. In: BD Sleeman, D Jones, eds. Ordinary and Partial Differential Equations, Vol. 1151 of Lecture Notes in Mathematics, Springer-Verlag, Berlin.
Rinzel J (1987) A formal classification of bursting mechanisms in excitable systems. In: E Teramoto, M Yamaguti, eds. Mathematical Topics in Population Biology, Morphogenesis, and Neurosciences, Vol. 71 of Lecture Notes in Biomathematics, Springer-Verlag, Berlin.
Rinzel J, Ermentrout GB (1989) Analysis of neural excitability and oscillations. In: C Koch, I Segev, eds. Methods in Neuronal Modeling, MIT Press, Cambridge MA.
Rinzel J, Lee YK (1987) Dissection of a model for neuronal parabolic bursting. Journal of Mathematical Biology 25:653–675.
Rowat PF, Selverston AI (1993) Modeling the gastric mill central pattern generator of the lobster with a relaxation-oscillator network. Journal of Neurophysiology 70:1030–1053.
Rush ME, Rinzel J (1994) Analysis of bursting in a thalamic neuron model. Biological Cybernetics 71:281–291.
Sherman A, Rinzel J, Keizer J (1988) Emergence of organized bursting in clusters of pancreatic β-cells by channel sharing. Biophysical Journal 54:411–425.
Skinner FK, Kopell N, Marder E (1994) Mechanisms of oscillation and frequency control in reciprocally inhibitory model neural networks. Journal of Computational Neuroscience 1:69–87.
Smolen P, Terman D, Rinzel J (1993) Properties of a bursting model with two slow inhibitory variables. SIAM Journal of Applied Mathematics 53:861–892.
Strumwasser F (1971) The cellular basis of behavior in Aplysia. Journal of Psychiatric Research 8:237–257.
Wilson WA (1982) Patterned bursting discharge of invertebrate neurons. In: DO Carpenter, ed. Cellular Pacemakers. Vol. 1, J. Wiley, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butera, R.J., Clark, J.W., Byrne, J.H. et al. Dissection and reduction of a modeled bursting neuron. J Comput Neurosci 3, 199–223 (1996). https://doi.org/10.1007/BF00161132
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00161132