Abstract
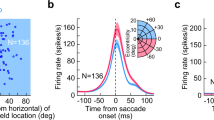
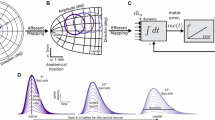
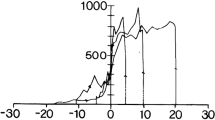
Saccade-related burst neurons (SRBNs) in the monkey superior colliculus (SC) have been hypothesized to provide the brainstem saccadic burst generator with the dynamic error signal and the movement initiating trigger signal. To test this claim, we performed two sets of open-loop simulations on a burst generator model with the local feedback disconnected using experimentally obtained SRBN activity as both the driving and trigger signal inputs to the model. First, using neural data obtained from cells located near the middle of the rostral to caudal extent of the SC, the internal parameters of the model were optimized by means of a stochastic hillclimbing algorithm to produce an intermediate-sized saccade. The parameter values obtained from the optimization were then fixed and additional simulations were done using the experimental data from rostral collicular neurons (small saccades) and from more caudal neurons (large saccades); the model generated realistic saccades, matching both position and velocity profiles of real saccades to the centers of the movement fields of all these cells. Second, the model was driven by SRBN activity affiliated with interrupted saccades, the resumed eye movements observed following electrical stimulation of the omnipause region. Once again, the model produced eye movements that closely resembled the interrupted saccades produced by such simulations, but minor readjustment of parameters reflecting the weight of the projection of the trigger signal was required. Our study demonstrates that a model of the burst generator produces reasonably realistic saccades when driven with actual samples of SRBN discharges.
Similar content being viewed by others
References
Arai K, Keller EL, Edelman JA (1994) Two-dimensional neural network model of the primate saccadic system. Neural Networks 7:1115–1135
Büttner-Ennever JA, Büttner U (1988) The reticular formation. In: Büttner-Ennever JA (eds) Neuroanatomy of the oculomotor system. Elsevier, Amsterdam, pp 119–176
Cynader M, Berman N (1972) Receptive-field organization of monkey superior colliculus. J Neurophysiol 35:187–201
Edwards SB, Henkel CK (1978) Superior colliculus connections with the extraocular motor nuclei in the cat. J Comp Neurol 179:451–468
Fuchs AF, Kaneko CRS, Scudder CA (1985) Brainstem control of saccadic eye movements. Annu Rev Neurosci 8:307–337
Gandhi NJ, Keller EL, Hartz KE (1994) Interpreting the role of collicular buildup neurons in saccadic eye movement control. Soc Neurosci Abstr 20:141
Glimcher PW, Sparks DL (1993) Effects of low-frequency stimulation of the superior colliculus on spontaneous and visually guided saccades. J Neurophysiol 69:953–964
Guitton D (1991) Control of saccadic eye and gaze movements by the superior colliculus and basal ganglia. In: Carpenter RHS (eds) Eye movements. CRC Press, Boca Raton, Fl, pp 244–276
Harris LR (1980) The superior colliculus and movements of the head and eye in cats. J Physiol (Lond) 300:367–391
Istvan PJ, Dorris MC, Munoz DP (1994) Functional identification of neurons in the monkey superior colliculus that project to the paramedian pontine reticular formation. Soc Neurosci Abstr 20:141
Jürgens R, Becker W, Kornhuber HH (1981) Natural and drug induced variations of velocity and duration of human saccadic eye movements: evidence for control of a neural pulse generator by local feedback. Biol Cybern 39:87–96
Keller EL (1977) Control of saccadic eye movements by midline brainstem neurons. In: Baker R, Berthoz A (eds) Control of gaze by brainstem neurons. Amsterdam, Elsevier
Keller EL (1979) Colliculo-reticular organization in the oculomotor system. Prog Brain Res 50:725–734
Keller EL (1981) Brainstem mechanisms in saccadic control. In: Fuchs AF, Becker W (eds) Progress in oculomotor research. New York, Elsevier/North-Holland
Keller EL, Edelman JA (1993) Effect of electrical stimulation in the omnipause region on cells in the superior colliculus of the monkey. Soc Neurosci Abstr 19:857
Keller EL, Edelman JA (1994) Use of the interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol 72: 2754–2770
King WM, Fuchs AF (1977) Neuronal activity in mesencephalon related to vertical eye movements. In: Baker R, Berthoz A (eds) Control of gaze by brainstem neurons. Amsterdam, Elsevier
Kirkpatrick S, Gelatt CD, Vecchi MP (1983) Optimization by simulated annealing. Science 20:671–680
Lefèvre P, Galiana HL (1992) Dynamic feedback to the superior colliculus in a neural network model of the gaze control system. Neural Networks 5:871–900
Massone LE (1994) A neural-network system for control of eye movements: basic mechanisms. Biol Cybern 71:293–305
Moschovakis AK (1994) Neural network simulations of the primate oculomotor system. I. The vertical saccadic burst generator. Biol Cybern 70:291–302
Moschovakis AK, Highstein SM (1994) The anatomy and physiology of primate neurons that control rapid eye movements. Annu Rev Neurosci 17:465–488
Moschovakis AK, Karabelas AB, Highstein SM (1988) Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol 60:263–302
Munoz DP, Wurtz RH (1993a) Interactions between fixation and saccade neurons in primate superior colliculus. Soc Neurosci Abstr 19:787
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70:559–575
Munoz D, Guitton D, Pelisson D (1991) Control of orienting gaze shifts by tecto-reticulo-spinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol 66:1642–1666
Optican LM (1994) Control of saccade trajectory by the superior colliculus. In: Fuchs AF, Brandt T, Büttner U, Zee DS (eds) Contemporary ocular, motor and vestibular research: a tribute to David A. Robinson. Georg Thieme, Stuttgart
Optican LM, Miles FA (1985) Visually induced adaptive changes in primate saccadic oculomotor control signals. J Neurophysiol 54:940–958
Richmond BJ, Optican LM (1987) Temporal encoding of two-dimensional patterns single units of primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol 57:147–161
Scudder CA (1988) A new local feedback model of the saccadic burst generator. J Neurophysiol 59:1455–1475
Scudder CA, Highstein S, Karabelas T, Moschovakis A (1989) Anatomy and physiology of long-lead burst neurons. Soc Neurosci Abstr 15:238
Sparks DL, Hartwich-Young R (1989) The deep layers of the superior colliculus. Oculomotor Res 3:213–255
Sparks DL, Holland R, Guthrie BL (1976) Size and distribution of movement fields in the monkey superior colliculus. Brain Res 113:21–34
Straschill M, Hoffmann KP (1970) Activity of movement sensitive neurons of the cat's tectum opticum during spontaneous eye movements. Exp Brain Res 11:318–326
Straschill M, Schick F (1977) Discharge of superior colliculus neurons uring head and eye movements of the alert cat. Exp Brain Res 27:131–141
Van Gisbergen JAM, Robinson DA, Gielen S (1981) A quantitative analysis of generation of saccadic eye movements by burst neuron. J Neurophysiol 45:417–441
Van Opstal AJ, Van Gisbergen JAM (1987) Skewness of saccadic velocity profiles: a unifying parameter for normal and slow saccades. Vision Res 27:731–745
Van Opstal AJ, Kappen H (1993) A two-dimensional ensemble coding model for spatial-temporal transformation of saccades in monkey superior colliculus. Network 4:19–38
Waitzman DA, Ma TP, Optican LM, Wurtz RH (1991) Superior colliculus mediate the dynamic characteristics of saccades. J Neurophysiol 66:1716–1737
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Das, S., Gandhi, N.J. & Keller, E.L. Open-loop simulations of the primate saccadic system using burst cell discharge from the superior colliculus. Biol. Cybern. 73, 509–518 (1995). https://doi.org/10.1007/BF00199543
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199543