Abstract
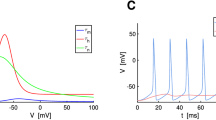
Hodgkin-Huxley-type models mimick the electrical behavior of excitable membranes quite realistically. However, inclusion of many different ionic channels into such a model yields a highly complex set of differential equations. In this paper a reduction of a “full” Hodgkin-Huxley-type model based on voltage-clamp data from small rat neurons in the supraoptic nucleus area is introduced. It was found that two of the ionic channel gating variables of the full model preserved a rather close relationship during simulations. This allowed to express one of these gating variables in terms of the other one thus reducing the number of differential equations the model is based on. The behavior of the reduced model was very similar to that of the full model. In particular, important physiological features as spike shape and constant-input-to-interspike-interval relationship were (almost) identical in the full and the reduced model.
Similar content being viewed by others
References
Av-Ron E, Parnas H, Segel AL (1991) A minimal biophysical model for an excitable and oscillatory neuron. Biol Cybern 65:487–500
Awiszus F (1990a) Effects of a slow potassium permeability on repetitive activity of the frog node of Ranvier. Biol Cybern 63:155–159
Awiszus F (1990b) Effects of a paranodal potassium permeability on repetitive activity of mammalian myelinated nerve fiber models. Biol Cybern 64:69–76
Awiszus F (1991) The influence of an unmyelinated terminal on repetitive firing of a mammalian receptor afferent fiber. Biol Cybern 64:421–427
Bawa P, Calancie B (1983) Repetitive doublets in human flexor carpi radialis muscle. J Physiol (London) 339:123–132
Blatz AL, Magleby KL (1987) Calcium-activated potassium channels. Trends Neurosci 10:463–467
Cobbett P, Legendre P, Mason WT (1989) Characterization of three types of potassium current in cultured neurones of rat supraoptic nucleus area. J Physiol (London) 410:443–462
Cobbett P, Mason WT (1987) Whole cell voltage clamp recordings from cultured neurons of the supraoptic area of neonatal rat hypothalamus. Brain Res 409:175–180
Cole KS (1968) Membranes, ions and impulses. University of California Press, Berkeley
Connor JA (1978) Slow repetitive activity from fast conductance changes in neurons. Fed Proc 37:2139–2145
Connor JA, Stevens CF (1971) Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol (London) 213:31–53
Connor JA, Walter D, McKown R (1977) Neural repetitive firing. Modifications of the Hodgkin-Huxley axon suggested by experimental results from crustacean axons. Biophys J 18:81–102
Finkel AS, Redman SJ (1983) The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol (London) 342:615–632
Frankenhaeuser B, Moore LE (1963) The effect of temperature on the sodium and potassium permeability changes in myelinated nerve fibres of Xenopus Laevis. J Physiol (London) 169:431–437
Hille B (1984) Ionic channels of excitable membranes. Sinauer, Sunderland Massachusetts
Hindmarsh JL, Rose RM (1982) A model of the nerve impulse using two first-order differential equations. Nature 296:162–164
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (London) 117:500–544
Kolb H-A (1990) Potassium channels in excitable and non-excitable cells. Rev Physiol Biochem Pharmacol 115:51–91
Miller RJ (1987) Multiple calcium channels and neuronal function. Science 235:46–52.
Nobel D (1966) Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev 46:1–50
Patlak J (1991) Molecular kinetics of voltage-dependent Na+ channels. Physiol Rev 71:1047–1080
Rall W (1989) Cable Theory for dendritic neurons. In: Koch C, Segev I (eds) Methods in neuronal modeling. MIT Press, Massachusetts, pp 9–62
Rose RM, Hindmarsh JL (1989) The assembly of ionic currents in a thalamic neuron. I. The three dimensional model. Proc R Soc Lond B 237:267–288
Schwarz JR (1986) The effect of temperature on Na currents in rat myelinated nerve fibers. Pflügers Arch 406:397–404
Schwarz JR, Eikhof G (1987) Na currents and action potentials in rat myelinated nerve fibres at 20 and 37° C. Pflügers Arch 409–569–577
Scott RH, Pearson HA, Dolphin AC (1991) Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol 36:485–520
Yamada WM, Koch C, Adams PR (1989) Multiple channels and calcium dynamics. In: Koch C, Segev I (eds) Methods in neuronal modeling. MIT Press, Massachusetts, pp 97–133
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Awiszus, F. Reduction of a Hodgkin-Huxley-type model for a mammalian neuron at body temperature. Biol. Cybern. 67, 427–432 (1992). https://doi.org/10.1007/BF00200986
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00200986