Abstract
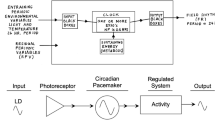
A coupled circadian oscillator model for the insect photoperiodic clock is described which consists of a hierarchically arranged pacemaker and slave. The pacemaker is self-sustained, temperature compensated, and entrainable by the light cycle; the slave is a damping oscillation receiving entrainment from two sources, from the pacemaker via a coupling factor, and also directly from the light. The damping slave oscillation is seen as the “photoperiodic oscillator”, equivalent to that proposed earlier by Lewis and Saunders (1987). The present simulations describe the effect of the strength of the coupling factor between hypothetical short- and long-period pacemaker oscillations (modelled on the “clock” mutants per sand per L2in Drosophila melanogaster) and a slave oscillation with a period of about 24 hours. The output is presented in terms of photoperiodic response curves and Nanda-Hamner, or resonance, plots. With a high coupling strength, the pacemakers strongly entrain the slave, but with a low coupling strength the slave's properties are more evident. The model is presented as a possible explanation for recent ovarian diapause data in D. melanogaster “clock” mutants (Saunders 1990), but also as a more general model for the role of the insect circadian system in seasonal time measurement.
Similar content being viewed by others
Abbreviations
- T :
-
The period of the experimental light-dark cycle in hours
- τ:
-
The free-running (unentrained) period of the circadian oscillation, in hours
- CNL:
-
The critical night length, the amount of darkness in a 24 hour period which induces 50% of a population to enter diapause
- CDL:
-
The critical day length, the reciprocal of CNL, i.e. if CNL = 10 h then CDL = (24 − 10) = 14 h
- PPRC:
-
Photoperiodic response curve
- PPTM:
-
Photoperiodic time measurement, the measurement of either day length or night length, the amount of light (or dark) being used as a signal which allows individuals to “tell” what time of year it is and to develop in a seasonally appropriate way
- IPI:
-
Inter-peak interval, the length of time between peaks in a resonance plot, corresponding to the endogeneous period of the photoperiodic rhythm
- φ i :
-
The photoinducible phase, a component of the external coincidence model of the photoperiodic clock. When illuminated during entrainment φ iinduces development in one way (non-diapause) whereas a different developmental path is taken (diapause) if this phase point is not illuminated Pittendrigh (1966)
- P s :
-
The model photoperiodic system containing a short period central oscillator, or pacemaker
- P L :
-
The model photoperiodic system containing a long period central oscillator, or pacemaker
- c pace :
-
Hypothetical oscillating chemical of the pacemaker in the coupled model
- c slave :
-
Ditto for the slave
- INDSUM:
-
Induction sum, the accumulated value of the diapause inducing titre produced when φ iis not illuminated (Lewis and Saunders 1987)
- CF:
-
The coupling strength between the pacemaker and the slave
- SR:
-
Synthesis rate of oscillating chemical c
- UB:
-
Maximum rate of synthesis of c, the Upper Bound
- TD:
-
The time delay incorporated into the negative feedback control of the level of c
References
Bowen MF, Saunders DS, Bollenbacher WE, Gilbert LI (1984) In vitro re-programming of the photoperiodic clock in an insect brainretrocerebral complex. Proc Natl Acad Sci USA 81:5881–5884
Bünning E (1936) Die Endogene Tagesrhythmik als Grundlage der Photoperiodischen Reaktion. Ber dt Bot Ges 54:590–607
Dowse HB, Hall JC, Ringo JM (1987) Circadian and ultradian rhythms in period mutants of Drosophila melanogaster. Behav Genet 17:19–35
Dowse HB, Ringo JM (1987) Further evidence that the circadian clock in Drosophila is a population of coupled ultradian oscillators. J Biol Rhythms 2:65–76
Follett BK (1982) Photoperiodic physiology in animals. In: Brady J (ed) Biological timekeeping, Society of Experimental Biology Series 14:83–100, Cambridge University Press, Cambridge, England
Hall JC, Rosbash M (1987) Genetic and molecular analysis of biological rhythms. J Biol Rhythms 2:153–178
Hall JC, Kyriacou CD (1990) Genetics of biological rhythms in Drosophila. Adv Insect Physiol 22:221–298
Handler AM, Konopka RJ (1979) Transplantation of a circadian pacemaker in Drosophila. Nature 279:236–238
Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 68:2112–2116
Konopka RJ, Pittendrigh CS, Orr D (1989) Reciprocal behaviour associated with the altered homeostasis and photosensitivity of Drosophila clock mutants, J. Neurogenetics 6:1–10
Lankinen P (1986) Geographical variation in circadian eclosion rhythm and photoperiodic adult diapause in Drosophila littoralis. J Comp Physiol 159:123–142
Lewis RD, Saunders DS (1987) A damped circadian oscillator model of an insect photoperiodic clock I. Description of the model based on a feedback control system. J Theoret Biol 128:47–59
Nanda KK, Hamner KC (1958) Studies on the nature of the endogeneous rhythm affecting photoperiodic response of biloxi soy bean. Bot Gaz 120:14–25
Pittendrigh CS (1958) Perspectives in the study of biological clocks. In: Buzzati-Traverso AA (ed) Perspectives in marine biology. University of California Press, USA pp 239–268
Pittendrigh CS (1966) The circadian oscillation in Drosophila pseudoobscura pupae: a model for the photoperiodic clock. Z Pflanzenphys 54:275–307
Pittendrigh CS (1972) Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci USA 69:2734–2737
Pittendrigh CS (1981) Circadian organization and the photoperiodic phenomena. In: Follett BK, Follett DE (ed) Biological clocks in seasonal reproductive cycles. John Wright and Sons Ltd. Bristol, UK pp 1–35
Pittendrigh CS, Bruce VG (1957) An oscillator model for biological clocks. In: Rudnick D (ed) Rhythmic and synthetic processes in growth. Princeton pp 75–109
Pittendrigh CS, Bruce VG (1959) Daily rhythms as coupled oscillator systems and their relation to thermoperiodism and photoperiodism. In: Withrow RB (ed) Photoperiodism and related phenomena in plants and animals. Am Ass Adv Sci, Washington, USA pp 475–505
Saunders DS (1973) The photoperiodic clock in the flesh-fly, Sar-cophaga argyrostoma J Insect Physiol 19:1941–54
Saunders DS (1981) Insect photoperiodism: the clock and the counter. Physiol Entomol 6:99–116
Saunders DS (1982) Photoperiodic induction of pupal diapause in Sarcophaga argyrostoma: Temperature effects on circadian resonance. J. Insect Physiol 28:305–310
Saunders DS (1990) The circadian basis of ovarian diapause regulation in Drosophila melanogaster: is the period gene causally involved in photoperiodic time measurement? J Biol Rhythms 5:315–331
Saunders DS, Henrich VC, Gilbert LI (1989) Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc Natl Acad Sci USA 86:3748–3752
Saunders DS, Gilbert LI (1990) Regulation of ovarian diapause in Drosophila melanogaster by photoperiod and moderately low temperature. J Insect Physiol 36:195–200
Saunders DS, Lewis RD (1987) A damped circadian oscillator model of an insect photoperiodic clock III. Circadian and “hourglass” responses. J Theoret Biol 128:73–85
Saunders DS, Richards DS, Applebaum SW, Ma M, Gilbert LI (1990) Photoperiodic diapause in Drosophila melanogaster involves a block to the juvenile hormone regulation of ovarian maturation. Gen Comp Endocrin 79:174–184
Smith RF, Konopka RJ (1981) Circadian clock phenotypes and chromosome aberrations with a breakpoint at the per locus. Mol Gen Genet 183: 243–251
Vaz Nunes M, Lewis RD, Saunders DS (1991a) A coupled oscillator feedback system as a model for the photoperiodic clock in insects and mites I. The basic control system as a model for circadian rhythms. J. Theoret Biol 152:287–298
Vaz Nunes M, Saunders DS, Lewis RD (1991b) A coupled oscillator feedback system as a model for the photoperiodic clock in insects and mites II. simulations of periodic responses. J Theoret Biol 152:299–317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gillanders, S.W., Saunders, D.S. A coupled pacemaker-slave model for the insect photoperiodic clock: interpretation of ovarian diapause data in Drosophila melanogaster . Biol. Cybern. 67, 451–459 (1992). https://doi.org/10.1007/BF00200989
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00200989