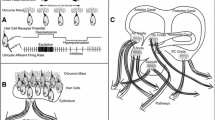
Abstract
It has been recently demonstrated that some primary otolith afferents and most otolith-related vestibular nuclei neurons encode two spatial dimensions that can be described by two vectors in temporal and spatial quadrature. These cells are called broadly-tuned neurons. They are characterized by a non-zero tuning ratio which is defined as the ratio of the minimum over the maximum sensitivity of the neuron. Broadly-tuned neurons exhibit response gains that do not vary according to the cosine of the angle between the stimulus direction and the cell's maximum sensitivity vector and response phase values that depend on stimulus orientation. These responses were observed during stimulation with pure linear acceleration and can be explained by spatio-temporal convergence (STC) of primary otolith afferents and/or otolith hair cells. Simulations of STC of the inputs to primary otolith afferents and vestibular nuclei neurons have revealed interesting characteristics: First, in the case of two narrowly-tuned input signals, the largest tuning ratio is achieved when the input signals are of equal gain. The smaller the phase difference between the input vectors, the larger the orientation differences that are required to produce a certain tuning ratio. Orientation and temporal phase differences of 30–40° create tuning ratios of approximately 0.10–0.15 in target neurons. Second, in the case of multiple input signals, the larger the number of converging inputs, the smaller the tuning ratio of the target neuron. The tuning ratio depends on the number of input units, as long as there are not more than about 10. For more than 10–20 input vectors, the tuning ratio becomes almost independent of the number of inputs. Further, if the inputs comprise two populations (with different gain and phase values at a given stimulus frequency), the largest tuning ratio is obtained when the larger population has a smaller gain. These findings are discussed in the context of known anatomical and physiological characteristics of innervation patterns of primary otolith afferents and their possible convergence onto vestibular nuclei neurons.
Similar content being viewed by others
References
Angelaki DE (1991a) Dynamic polarization vector of spatially neurons. IEEE Trans Biom Eng 38:1053–1060
Angelaki DE (1991b) Spatio-temporal convergence (STC) in otolith neurons. Soc Neurosci (abstr) 17:316
Angelaki DE (1992) Vestibular neurons encoding two-dimensional linear acceleration assist in the estimation of rotational velocity during off-vertical axis rotation. Ann NY Acad Sci (in press)
Angelaki DE, gnBush GA, Perachio AA (1992) A model for the characterization of the spatial properties in vestibular neurons. Biol Cybern 66:231–240
Baird RA (1992) Morphological and electrophysiological properties of hair cells in the bullfrog utriculus. Ann NY Acad Sci (in press)
Baker J, Goldberg J, Hermann G, Peterson B (1984) Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res 294:138–143
Bush GA, Perachio AA, Angelaki DE (1992) Quantification of different classes of canal-related vestibular nuclei neuron responses to linear acceleration. Ann NY Acad Sci (in press)
Chan YS, Cheung YM, Hwang JC (1987) Response characteristics of neurons in the cat vestibular nuclei during slow and constant velocity off-vertical axes rotations in the clockwise and counterclockwise rotations. Brain Res 406:294–301
Coreia MJ, Lang DG (1990) An electrophysiological comparison of solitary type I and type II vestibular hair cells. Neurosci Lett 116:106–111
Dickman JD, Angelaki DE, Correia MJ (1991) Response properties of gerbil otolith afferents to small angle pitch and roll tilts. Brain Res 556:303–310
Eatock RA, Corey DP, Hudspeth AJ (1987) Adaptation of mechanoelectric transduction in hair cells of the bullfrog sacculus. J Neurosci 7:2821–2836
Fernandez C, Goldberg JM, Abend WK (1972) Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurosphysiol 35:978–997
Fernandez C, Goldberg JM (1976a) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long duration centrifugal force. J Neurophysiol 39:970–984
Fernandez C, Goldberg JM (1976b) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force response relations. J Neurophysiol 39:985–995
Fernandez C, Goldberg JM (1976c) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39:996–1008
Fernandez C, Goldberg JM, Baird RA (1990) The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63:767–780
Goldberg JM, Desmadryl G, Baird R, Fernandez C (1990a) The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63:781–790
Goldberg JM, Desmadryl G, Baird R, Fernandez C (1990b) The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63:791–804.
Lindeman HH (1969) Studies on the morphology of the sensory regions of the vestibular apparatus. Ergeb Anat Entwickl-Gesch 42:1–113
Loe PR, Tomko DL, Werner G (1973) The neural signal of angular head position in primary afferent vestibular nerve axons. J Physiol 219:29–50
Lorente de No R (1926) Etudes sur l'anatomie et al physiologie du labyrinthe de l'oreille et du VIIIe nerf. Trabajos Lab Invest Biol Univ Madrid 24:53–153
Melvill Jones G, Milsum JH (1969) Neural response of the vestibular system to translational acceleration. In: Supplement to conference on systems analysis approach to neurophysiological problems, Brainerd MN [Suppl] pp 8–20
Ohmori H (1987) Gating properties of the mechanoelectrical transducer channel in the dissociated vestibular hair cell of the chick. J Physiol 387:589–609
Rennie KJ, Ashmore JF (1991) Ionic currents in isolated vestibular hair cells from the guinea-pig crista ampullaris. Hear Res 51:279–292
Ross MD, Rogers CM, Donovan KM (1986) Innervation patterns in rat saccular macula. Acta Otolaryngol (Stockh) 102:75–86
Schor RH, Miller AD, Tomko DL (1984) Responses to heat tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51:136–146
Shotwell SL, Jacobs R, Hudspeth AJ (1981) Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann NY Acad Sci 374:1–10
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Angelaki, D.E. Spatio-temporal convergence (STC) in otolith neurons. Biol. Cybern. 67, 83–96 (1992). https://doi.org/10.1007/BF00201805
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201805