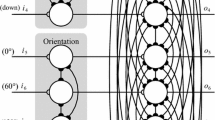
Abstract
Nonassociative learning is an important property of neural organization in both vertebrate and invertebrate species. In this paper we propose a neural model for nonassociative learning in a well studied prototypical sensory-motor scheme: the landing reaction of flies. The general structure of the model consists of sensory processing stages, a sensory-motor gate network, and motor control circuits. The paper concentrates on the sensory-motor gate network which has an agonist-antagonist structure. Sensory inputs to this circuit are transduced by chemical messenger systems whose dynamics include depletion and replenishment terms. The resulting circuit is a gated dipole anatomy and we show that it gives a good account of nonassociative learning in the landing reaction of the fly.
Similar content being viewed by others
References
Arnett DW (1972) Spatial and temporal integration properties of units in first optic ganglion of dipterans. J Neurophysiol 35:429–444
Beghi L, Saviolo N, Xausa E, Zanforlin M (1983) Perception of movement, the correlation model and the landing reaction of the flesh fly (Calliphorinae). Biol Cybern 46:81–91
Borst A (1986) Time course of the houseflies' landing response. Biol Cybern 54:379–383
Borst A (1989) Temporal processing of excitatory and inhibitory motion stimuli in the fly's landing system. Naturwissenschaften 76:531–534
Borst A, Bahde S (1986) What kind of movement detector is triggering the landing response of the housefly? Biol Cybern 55:59–69
Borst A, Bahde S (1987) Comparison between the movement detection systems underlying the optomotor and the landing response in the housefly. Biol Cybern 56:217–224
Borst A, Bahde S (1988a) Visual information processing in the fly's landing system. J Comp Physiol A 163:167–173
Borst A, Bahde S (1988b) Spatio-temporal integration of motion. A simple strategy for safe landing in flies. Naturwissenschaften 75:265–267
Braitenberg V, Taddei-Ferretti C (1966) Landing reaction of Musca domestica induced by visual stimuli. Naturwissenschaften 53:155
Brent R (1971) An algorithm with guaranteed convergence for finding a zero of a function. Comput J 14:422–425
Buchner E (1976) Elementary movement detectors in an insect visual system. Biol Cybern 24:85–101
Bullock D, Grossberg S (1988) Neural dynamics of planned arm movements: Emergent invariants and speed-accuracy properties during trajectory formation. Psychol Rev 95:49–90
Campbell FW, Maflei L (1979) Sloped visual motion. Nature 278:192
Chillemi S, Taddei-Ferretti C (1981) Landing reaction of Musca domestica. VI. Neurones responding to stimuli that elicit the landing response in the fly. J Exp Biol 94:105–118
Dennis J Jr, Schnabel R (1983) Numerical methods for unconstrained optimization and nonlinear equations. Prentice-Hall, New Jersey
Eckert H (1980) Orientation sensitivity of the visual movement detection system activating the landing response on the blowflies, Calliphora, and Phaenicia: A behavioural investigation. Biol Cybern 37:235–247
Eckert H, Hamdorf K (1980) Excitatory and inhibitory response components in the landing response of the blowfly, Calliphora erythrocephala. J Comp Physiol 138:253–264
Eckert H, Hamdorf K (1983) Does a homogeneous population of elementary movement detectors activate the landing response of blowflies, Calliphora erythrocephala? Biol Cybern 48:11–18
Fischbach K (1981) Habituation and sensitization of the landing response of Drosophila melanogaster. Naturwissenschaften 68:332
Fischbach KF, Bausenwein B (1988) Habituation and sensitization of the landing response of Drosophila melanogaster: II Receptive field size of habituating units. In: Hertting O, Spatz HC (eds) Modulation of synaptic transmission and plasticity in nervous systems. Springer, Berlin Heidelberg New York
Gaudiano P, Grossberg S (1991) Vector associative maps: Unsupervised real-time error-based learning and control of movement trajectories. Neural Networks 4:147–183
Gill P, Murray W (1976) Minimization subject to bounds on the variables. NPL Report NAC 72, National Physical Laboratory, England
Goodman LJ (1960) The landing responses of insects. I. The landing response of the fly, Lucilia sericata, and other Calliphorinae. J Exp Biol 37:854–878
Grossberg S (1972) A neural theory of punishment and avoidance. II. Quantitative theory. Math Biosci 15:253–285
Grossberg S (1988) Nonlinear neural networks: Principles, mechanisms, and architectures. Neural Networks 1:17–61
Grossberg S, Kuperstein M (1989) Neural dynamics of adaptive sensory-motor control. Pergamon Press, New York
Hausen K (1984) The lobula complex of the fly: Structure, functions and significance in visual behavior. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum Press, New York
Hull T, Enright W, Jackson K (1976) User's guide for DVERK — A subroutine for solving non-stiff ODE's. Department of Computer Science Technical Report 100, University of Toronto
Hunzelmann N, Spillman L (1984) Movement adaptation in the peripheral retina. Vision Res 24: 1765–1769
IMSL Math Library Manual (1987) Houston
Jackson K, Enright W, Hull T (1978) A theoretical criterion for comparing Runge-Kutta formulas. SIAM J Num Anal 15:618–641
Jansonius NM, van Harteren JH (1991) Fast temporal adaptation of on-off units in the first optic chiasm of the blowfly. J Comp Physiol A 168:631–637
Lipsitt LP (1990) Learning processes in the human newborn: Sensitization, habituation, and classical conditioning. In: Diamond A (ed) The development and neural based of higher cognitive functions. Ann NY Acad Sci 608
MacKay DM (1982) Anomalous perception of extrafoveal motion. Perception 11:359–360
Marcus EA, Carew TJ (1990) Ontogenetic analysis of learning in a simple system. In: Diamond A (ed) The development and neural bases of higher cognitive funcitons. Ann NY Acad Sci 608
Öğmen H (in press) Sensorial nonassociative learning and its implications for visual perception. In: Omidvar O (ed) Progress in neural networks, Ablex, New Jersey
Öğmen H (1992) On the developmental basis of self-organization and its active exploratory components. University of Houston Systems, Control, and Computing Technical Reports, No. 92-03
Öğmen H, Gagné S (1990a) Neural network architectures for motion perception and elementary motion detection in the fly visual system. Neural Networks 3:487–505
Öğmen H, Gagné S (1990b) Neural models for sustained and on-off units of insect lamina. Biol Cybern 63:51–60
Perez de Talens FA, Taddei-Ferretti C (1970) Landing reaction of Musca domestica: Dependence on dimensions and position of the stimulus. J Exp Biol 52:233–256
Piaget J (1967) La psychologie de l'intelligence. Armand Colin, Paris
Taddei-Ferretti C, Perez de Talens FA (1973a) Landing reaction of Musca domestica. III. Dependence on the luminous characteristics of the stimulus. Z Naturforsch 28c:568–578
Taddei-Ferretti C, Perez de Talens FA (1973b) Landing reaction of Musca domestica. IV. A. Monocular and binocular vision. B. Relationship between landing and optomotor reactions. Z Naturforsch 28c:579–592
Taddei-Ferretti C, Chillemi S, Contugno A (1980) Landing reaction of Musca domestica: Neural correlates of the landing reaction. Naturwissenschaften 67:101–102
Tinbergen J, Abeln R (1983) Spectral sensitivity in the landing blowfly. J Comp Physiol 150:319–328
Wagner H (1982) Flow-field variables trigger landing in flies. Nature 297:147–148
Waldvogel FM, Fischback KF (1991) Plasticity of the landing reaction of Drosophila melanogaster. J Comp Physiol A 169:323–330
Wehrhahn K, Hausen K, Zanker J (1981) Is the landing response of the housefly (Musca) driven by motion of a flow-field? Biol Cybern 41:91–99
Wittekind WC, Spatz HC (1988) Habituation of the landing response of Drosophila. In: Hertting G, Spatz HC (eds) Modulation of synaptic transmission and plasticity in nervous systems. Springer, Berlin Heidelberg New York
Author information
Authors and Affiliations
Additional information
Supported by a grant from the National Institute of Mental Health
About this article
Cite this article
Öğmen, H., Moussa, M. A neural model for nonassociative learning in a prototypical sensory-motor scheme: the landing reaction in flies. Biol. Cybern. 68, 351–361 (1993). https://doi.org/10.1007/BF00201860
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201860