Summary
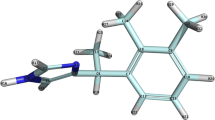
The recent elucidation of the primary structure of the cell membrane-boundβ-adrenoceptor has prompted us to explore putative ligand binding sites on this physiologically important receptor. By minimizing the energies of the ‘prototype’ ligand propranolol, (part of) the receptor and the proposed ligand-receptor complex with the aid of force field and quantum chemical calculations, we identified amino acid residue Trp313 as a highly probable candidate for interaction with the aromatic moiety of propranolol. The charge distribution on the indole nucleus of anotherβ-blocker, pindolol, with higher affinity for theβ-adrenoceptor, enables an even stronger interaction with the tryptophan residue. The carboxylic amino acid residue Glu306, located near the extracellular space of the cell membrane, interacts favorably with the positively charged nitrogen atom in the aliphatic side chain of the ligands. Finally, this putative model is discussed in the light of recent findings in mutagenesis studies, and compared to other ideas with respect to ligand-receptor interactions.
Similar content being viewed by others
References
Lefkowitz, R.J., Stadel, J.M. and Caron, M.G., Annu. Rev. Biochem., 52 (1983) 159–186.
Lands, A.M., Arnold, A., McAuliff, J.P., Luduena, F.P. and Brown Jr., T.G., Nature, 214 (1967) 597–598.
Lefkowitz, R.J., Caron, M.G., Michel, T. and Stadel, J.M., Fed. Proc., 41 (1982) 2664–2670.
Ijzerman, A.P., Bultsma, T., Timmerman, H. and Zaagsma, J. Naunyn-Schmied. Arch. Pharmacol., 327 (1984) 293–298.
Ijzerman, A.P., Dorlas, R., Aué, G.H.J., Bultsma, T. and Timmerman, H., Biochem. Pharmacol., 34 (1985) 2883–2890.
Cherksey, B.D., Murphy, R.B. and Zadunaisky, J.A., Biochemistry, 20 (1981) 4278–4283.
Ijzerman, A.P., Aué, G.H.J., Bultsma, T., Linschoten, M.R. and Timmerman, H., J. Med. Chem., 28 (1985) 1328–1334.
Linschoten, M.R., Bultsma, T., Ijzerman, A.P. and Timmerman, H., J. Med. Chem., 29 (1986) 278–286.
Kobilka, B.K., Dixon, R.A.F., Frielle, T., Dohlman, H.G., Bolanowski, M.A., Sigal, I.S., Yang-Feng, T.L., Francke, U., Caron, M.G. and Lefkowitz, R.J., Proc. Natl. Acad. Sci. U.S.A., 84 (1987) 46–50.
Dixon, R.A.F., Kobilka, B.K., Strader, D.J., Benovic, J.L., Dohlman, H.G., Frielle, T., Bolanowski, M.A., Bennett, C.D., Rands, E., Diehl, R.E., Mumford, R.A., Slater, E.E., Sigal, I.S., Caron, M.G., Lefkowitz, R.J. and Strader, C.D., Nature 321 (1986) 75–79.
Yarden, Y., Rodriguez, H., Wong, S. K.-F., Brandt, D.R., May, D.C., Burnier, J., Harkins, R.N., Chen, E.Y., Ramachandran, J., Ullrich, A. and Ross, E.M., Proc. Natl. Acad. Sci. U.S.A., 83 (1986) 6795–6799.
Dohlman, H.G., Caron, M.G. and Lefkowitz, R.J., Biochemistry, 26 (1987) 2657–2664.
Dixon, R.A.F., Sigal, I.S., Rands, E., Register, R.B., Candelore, M.R., Blake, A.D. and Strader, C.D., Nature, 326 (1987) 73–77.
CHEM-X: Molecular modelling system, Chemical Design Ltd., Oxford, U.K.
QCPE-program No. 455, MOPAC: A General Molecular Orbital Package, Chemistry Department, Indiana University, Bloomington, IN, U.S.A.
Allinger, N.L. and Yuh, Y.H., QCPE-program No. 395, MM2.
Allinger, N.L. and Yuh, Y.H., QCPE-program Nos. 395,400, MM2P.
Del Re, G., Gavuzzo, E., Giglio, E., Lelj, F., Mazza, F. and Zappia, V., Acta Crystallogr., Sect. B33 (1977) 3289–3296.
Easson, L.H. and Stedman, E., Biochem. J., 27 (1933) 1257–1266.
Albert, A., Selective Toxicity and Related Topics, 4th ed., Methuen & Co Ltd., London, 1968, pp. 182–187.
Bilezikian, J.P., Dornfeld, A.M. and Gammon, D.E., Biochem. Pharmacol., 27 (1978) 1455–1461.
Andrews, P.R., Craik, D.J. and Martin, J.L., J. Med. Chem., 27 (1984) 1648–1657.
Oseroff, A.R. and Callender, R.H., Biochemistry, 13 (1974) 4243–4248.
Applebury, M.L. and Hargrave, P.A., Vision Res., 26 (1987) 1881–1895.
Strader, C.D., Sigal, I.S., Register, R.B., Candelore, M.R., Rands, E. and Dixon, R.A.F., Proc. Natl. Acad. Sci. U.S.A. 84 (1987) 4384–4388.
Hollenberg, M.D., Trends Pharmacol. Sci., 8 (1987) 197–199.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ijzerman, A.P., van Vlijmen, H.W.T. A molecular graphics study exploring a putative ligand binding site of theβ-adrenoceptor. J Computer-Aided Mol Des 2, 43–53 (1988). https://doi.org/10.1007/BF01532052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01532052