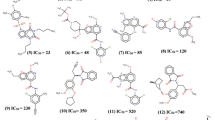
Summary
Modelling studies have been carried out on the phosphodiesterase (PDE) substrates, adenosine- and guanosine-3′5′-cyclic monophosphates, and on a number of non-specific and type III-specific phosphodiesterase inhibitors. These studies have assisted the understanding of PDE substrate differentiation and the design of potent, selective PDE type III inhibitors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Beavo, J.A., Hansen, R.S., Harrison, S.A., Hurwitz, R.L., Martins, T.J. and Mumby, M.C., Mol. Cell. Endocrinol., 28 (1982) 387–410.
Vinter, J.G., Davis, A. and Saunders, M.R., J. Comput. — Aided Mol. Design. 1 (1987) 31–51.
Reeves, M.L., Leigh, B.K. and England, P.J., Biochem. J., 241 (1987) 535–541.
Fitzakerley, G.V., Russell, J.C. and Wolfe, M.A., Eur. J. Biochem., 76 (1977) 601–605.
Yathindra, N. and Sundaralingam, M., Biochem. Biophys. Res. Commun., 56 (1974) 119–126.
Druyan, M.E., Sparagana, M. and Peterson, M.E., J. Cycl. Nucleotide Protein Phosphorylation Res., 2 (1976) 373–377.
Topal, M.D. and Fresco, J.R., Nature, 263 (1976) 285–289.
Chenon, M.T., Pugmire, R.J., Grant, D.M., Panzica, R.P. and Townsend, L.B., J. Am. Chem. Soc., 97 (1975) 4636–4642.
Kramer, G.L., Garst, J.E., Mitchell, S.S. and Wells, J.N., Biochemistry. 16 (1977) 3316–3321.
Coulson, C.J., Ford, R.E., Lunt, E., Marshall, S., Pain, D.L., Rogers, I.H. and Wooldridge, K.R.H., Eur. J. Med. Chem. 9 (1974) 313–320.
Robertson, D.W., Beedle, E.E., Swartzendruber, J.K., Jones, N.D., Elzey, T.K., Kauffmann, R.F., Wilson, H. and Hayes, J.S., J. Med. Chem., 29 (1986) 635–640.
Hertzig, J.W., Feile, K. and Ruegg, J.C., Arzneim.-Forsch. Drug Res., 31 (1981) 188–191.
Novelli, R. and Reeves, M.L., Unpublished.
Prout, C.K., Unpublished.
Cheng, Y.-C. and Prusoff, W.H., Biochem. Pharmacol., 22 (1973) 3099–3108.
Andrews, R.R., Craik, D.J. and Martin, J.L., J. Med. Chem., 27 (1984) 1648–1657.
Bristol, J.A., Sircar, I., Moos, W.H., Evans, D.B. and Weishaar, R.E., J. Med. Chem., 27 (1984) 1101–1103.
Sircar, I., Duell, B.L., Bobowski, G., Bristol, J.A. and Evans, D.B., J. Med. Chem., 28 (1985) 1405–1413.
Sircar, I., Duell, B.L., Cain, M.H., Burke, S.E. and Bristol, R.A., J. Med. Chem., 29 (1986) 2142–2148.
Weishaar, R.E., Caine, M.H. and Bristol, J.A., J. Med. Chem., 28 (1985) 537–545.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davis, A., Warrington, B.H. & Vinter, J.G. Strategic approaches to drug design. II. Modelling studies on phosphodiesterase substrates and inhibitors. J Computer-Aided Mol Des 1, 97–119 (1987). https://doi.org/10.1007/BF01676955
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01676955