Summary
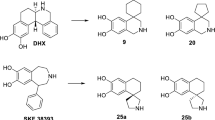
A series of apomorphine congeners has been studied with respect to their ability to mimic the structural requirements of the dopamine pharmacophore in the potent and stereoselective dopamine receptor agonist (R)-apomorphine. Conformational energies of the mimicking structures calculated by molecular mechanics (MMP2) correlate well with the observed biological activities.
Similar content being viewed by others
References
Saari, W.S., King, S.W. and Lotti, V.J., J. Med. Chem., 16 (1973) 171–172.
Giesecke, J., Acta Crystallogr., Sect. B, 29 (1973) 1785–1791.
Kaiser, C. and Jain, T., Med. Res. Q. 5 (1985) 145–229.
Burkert, U. and Allinger, N.L., Molecular Mechanics, American Chemical Society, Washington, D.C., 1982. (The MM2/MMP2 programs are available from the Quantum Chemistry Program Exchange, University of Indiana, Bloomington, IN 47405 and from Molecular Design Ltd., 2132 Farallon Drive, San Leandro, CA 94577.)
Liljefors, T., J. Mol. Graph., 1 (1983) 111–117.
Von der Lieth, C. W., Carter, R. E., Dolata, D.P. and Liljefors, T., J. Mol. Graph., 2 (1984) 117–123.
Molecular modeling system SYBYL, TRIPOS Associates, Inc., 6548 Clayton Road, St. Louis, MO 63117.
Andrews, P.R., Lloyd, E.J., Martin, J.L. and Munro, S.L.A., J. Mol. Graph., 4 (1986) 41–45.
Lloyd, J.E. and Andrews, P.R., J. Med. Chem. 29 (1986) 453–462.
Cannon, J.G., Borgmann, R.J., Aleem, M.A. and Long, J.P., J. Med. Chem., 16 (1973) 219–224.
Cannon, J.G., Smith, R.V., Aleem, M.A. and Long, J.P., J. Med. Chem., 18 (1975) 108–110.
Cannon, J.G., Kim, J.C., Aleem, M.A., and Long, J.P., J. Med. Chem., 15 (1972) 348–350.
Ginos, J.Z., Cotzias, G.C., Tolosa, E., Tang, L.C. and LoMonte, A., J. Med. Chem., 18 (1975) 1194–1200.
Cannon, J.G., Perez, Z., Long, J.P., Rusterholz, D.B., Flynn, J.R., Costall, B., Fortune, D.H. and Naylor, R.J., J. Med. Chem., 22 (1978), 901–907.
Seeman, P., Titeler, M., Tedesco, J., Weinreich, P. and Sinclair, D., Adv. Biochem. Psychopharmacol., 19 (1978) 167–176.
Misiorny, A., Ross, S.B. and Stjernstrom, N.E., Acta Pharm. Suecica. 14 (1977) 105–112.
Sandoz GmbH, Derwent No. 83-790018/42, DE Patent 3312-420-A, Oct. 13, 1983.
Berney, D., Petcher, T.J., Schultz, J., Weber, H.P. and White, T.G., Experentia, 31 (1975) 1327–1328.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pettersson, I., Liljefors, T. Structure-activity relationships for apomorphine congeners. Conformational energies vs. biological activities. J Computer-Aided Mol Des 1, 143–152 (1987). https://doi.org/10.1007/BF01676958
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01676958