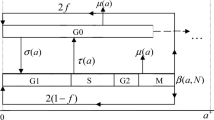
Abstract
We present a nonlinear first-order hyperbolic partial differential equation model to describe age-structured tumor cell populations with proliferating and quiescent phases at the avascular stage in vitro. The division rate of the proliferating cells is assumed to be nonlinear due to the limitation of the nutrient and space. The model includes a proportion of newborn cells that enter directly the quiescent phase with age zero. This proportion can reflect the effect of treatment by drugs such as erlotinib. The existence and uniqueness of solutions are established. The local and global stabilities of the trivial steady state are investigated. The existence and local stability of the positive steady state are also analyzed. Numerical simulations are performed to verify the results and to examine the impacts of parameters on the nonlinear dynamics of the model.












Similar content being viewed by others
References
Akimenko, V., Anguelov, R.: Steady states and outbreaks of two-phase nonlinear age-structured model of population dynamics with discrete time delay. J. Biol. Dyn. 11(1), 75–101 (2016)
Alzahrani, E.O., Asiri, A., El-Dessoky, M.M., Kuang, Y.: Quiescence as an explanation of Gompertzian tumor growth revisited. Math. Biosci. 254, 76–82 (2014)
Alzahrani, E.O., Kuang, Y.: Nutrient limitations as an explanation of Gompertzian tumor growth. Discrete Contin. Dyn. Syst. Ser. B 21(2), 357–372 (2016)
Araujo, R.P., McElwain, D.L.S.: A history of the study of solid tumour growth: the contribution of mathematical modelling. Bull. Math. Biol. 66, 1039–1091 (2004)
Arino, O., Kimmel, M.: Asymptotic analysis of a cell-cycle model based on unequal division. SIAM J. Appl. Math. 47, 128–145 (1987)
Arino, O., Sánchez, E., Webb, G.F.: Necessary and sufficient conditions for asynchronous exponential growth in age structured cell populations with quiescence. J. Math. Anal. Appl. 215, 499–513 (1997)
Ayati, B.P., Webb, G.F., Anderson, R.A.: Computational methods and results for structured multiscale models of tumor invasion. SIAM Multiscale Model. Simul. 5(1), 1–20 (2006)
Bertalanffy, L.V.: Quantitative laws in metabolism and growth. Q. Rev. Biol. 32, 217–231 (1957)
Bi, P., Ruan, S., Zhang, X.: Periodic and chaotic oscillations in a tumor and immune system interaction model with three delays. Chaos 24, 023101 (2014)
Breward, C.J.W., Byrne, H.M., Lweis, C.E.: A multiphase model describing vascular tumour growth. Bull. Math. Biol. 01, 1–28 (2004)
Brikci, F.B., Clairambault, J., Ribba, B., Perthame, B.: An age-and-cyclin-structured cell population model for healthy and tumoral tissues. J. Math. Biol. 57(1), 91–110 (2008)
Busenberg, S.N., Iannelli, M., Thieme, H.R.: Global behavior of an age-sgructured epidemic model. SIAM J. Math. Anal. 22, 1065–1080 (1991)
Carlsson, J.: A proliferation gradient in three-dimensional colonies of cultured human glioma cells. Int. J. Cancer 20, 129–136 (1977)
Cherif, A., Dyson, J., Maini, P.K., Gupta, S.: An age-structured multi-strain epidemic model for antigenically diverse infectious diseases: a multi-locus framework. Nonlinear Anal. Real World Appl. 34, 275–315 (2017)
Congar, A.D., Ziskin, M.C.: Growth of mammalian multicellular tumour spheroids. Cancer Res. 43, 558–560 (1983)
Dyson, J., Villella-Bressan, R., Webb, G.F.: Asynchronous exponential growth in an age structured population of proliferating and quiescent cells. Math. Biosci. 177, 73–83 (2002)
Florian, J.A., Eiseman, J.L., Parker, R.S.: Accounting for quiescent cells in tumour growth and cancer treatment. IEE Proc. Syst. Biol. 152(4), 185–192 (2005)
Folkman, J.: Role of angiogenesis in tumour growth and metastases. Semin. Oncol. 29, 15–19 (2002)
Folkman, J., Cotran, R.: Relation of vascular proliferation to tumour growth. Int. Rev. Exp. Pathol. 16, 207–248 (1976)
Folkman, J., Hochberg, M.: Self-regulation of growth in three dimensions. Exp. Med. 138, 745–753 (1973)
Gabriel, P., Garbett, S.P., Quaranta, V., Tyson, D.R., Webb, G.F.: The contribution of age structure to cell population responses to targeted therapeutics. J. Theor. Biol. 311(21), 19–27 (2012)
Gurtin, M.E., Maccamy, R.C.: Non-linear age-dependent population dynamics. Arch. Ration. Mech. Anal. 54(3), 281–300 (1974)
Gyllenberg, M., Webb, G.F.: Age-size structure in populations with quiescence. Math. Biosci. 86, 67–95 (1987)
Gyllenberg, M., Webb, G.F.: Quiescence as an explanation of Gompertzian tumor growth. Growth Dev. Aging 53, 25–33 (1989)
Gyllenberg, M., Webb, G.F.: Asynchronous exponential growth of semigroups of nonlinear operators. J. Math. Anal. Appl. 167, 443–467 (1992)
Hartung, N., Mollard, S., Barbolosi, D., Benabdallah, A., Chapuisat, G., Henry, G., Giacometti, S., Iliadis, A., Ciccolini, J., Faivre, C., Hubert, F.: Mathematical modeling of tumor growth and metastatic spreading: validation in tumor-bearing mice. Cancer Res. 74, 6397–6407 (2014)
Hubbard, M.E., Byrne, H.M.: Multiphase modelling of vascular tumour growth in two spatial dimensions. J. Theor. Biol. 316, 70–89 (2013)
Inaba, H.: A semigroup approach to the strong ergodic theorem of the multi-state stable population process. Math. Popul. Stud. 1(1), 49–77 (1988)
Inaba, H.: Mathematical analysis of an age-structured SIR epidemic model with vertical transmission. Discrete Contin. Dyn. Syst. Ser. B 6(1), 69–96 (2006)
Laird, A.K.: Dynamics of tumor growth. Br. J. Cancer 18, 490–502 (1964)
Liotta, L.A., Saidel, G.M., Kleinerman, J.: Stochastic model of metastases formation. Biometrics 32, 535–550 (1976)
Liu, D., Ruan, S., Zhu, D.: Stable periodic oscillations in a two-stage cancer model of tumor and immune system interactions. Math. Biosci. Eng. 9(2), 347–368 (2012)
Lowengrub, J.S., Frieboes, H.B., Jin, F., Chuang, Y.L., Li, X., Macklin, P., Wise, S.M., Cristini, V.: Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23(1), R1–R9 (2010)
Newton, P.K., Mason, J., Bethel, K., Bazhenova, L.A., Nieva, J., Kuhn, P.: A stochastic Markov chain model to describe lung cancer growth and metastasis. PLoS ONE 7(4), e34637 (2013)
Orme, M.E., Chaplain, M.A.J.: A mathematical model of vascular tumour growth and invasion. Math. Comput. Model. 23(10), 43–60 (1996)
Pinho, S.T.R., Freedman, H.I., Nani, F.: A chemotherapy model for the treatment of cancer with metastasis. Math. Comput. Model. 36, 773–803 (2002)
Ramis-Conde, I., Chaplain, M.A.J., Anderson, A.R.A.: Mathematical modeling of cancer cell invasion of tissue. Math. Comput. Model. 47, 533–545 (2008)
Spinelli, L., Torricelli, A., Ubezio, P., Basse, B.: Modelling the balance between quiescence and cell death in normal and tumour cell populations. Math. Biosci. 202, 349–370 (2006)
Tan, W.Y.: A stochastic model for the formation of metastatic foci at distant sites. Math. Comput. Model. 12(9), 1093–1102 (1989)
Tyson, D.R., Garbett, S.P., Frick, P.L., Quaranta, V.: Fractional proliferation: a method to deconvolve cell population dynamics from single-cell data. Nat. Methods 9(9), 923–928 (2012)
Ward, J.P., King, J.R.: Mathematical modelling of avascular-tumour growth. IMA J. Math. Appl. Med. Biol. 14, 39–69 (1997)
Ward, J.P., King, J.R.: Mathematical modelling of avascular-tumour growth II: modelling growth saturation. IMA J. Math. Appl. Med. Biol. 16, 171–211 (1999)
Acknowledgements
The authors are grateful to the two anonymous reviewers and the handling editor for their helpful comments and suggestions which helped us in improving the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Trachette Jackson.
This research was supported by the National Natural Science Foundation of China (11401060, 11401117, 11401217, 11771168), the Basic and Advanced Research Project of Chongqing (cstc2016jcyjA0412), the Program of Chongqing Innovation Team Project in University (CXTDX201601022) and Chongqing Municipal Education Commission (KJ1600522, KJ1705136).
Existence and Uniqueness of Solutions
Existence and Uniqueness of Solutions
Consider a Banach space \(\mathbf {X}=L^1(0,a^+)\times L^1(0,a^+)\) endowed with the norm \(||\phi ||=||\phi _1||+||\phi _2||\) for \(\phi (a)=(\phi _1(a),\phi _2(a))^T\in \mathbf {X}\), where \(||\cdot ||\) is the norm in \(L^1\) and \(v^T\) is the transpose of the vector v.
Now we define a linear operator \(A: D(A)\subset \mathbf {X}\rightarrow \mathbf {X}\) by
The domain D(A) is given as
where \(L_+^1(0,a^+)\) denotes the positive cone of \(L^1(0,a^+)\) and \(AC[0,a^+]\) is the set of absolutely continuous functions on \([0,a^+)\), \(\phi _1(0)=2(1-f)\int _0^{a^+} \beta (a,N(t))\phi _1(a)\hbox {d}a\) and \(\phi _2(0)=2f\int _0^{a^+} \beta (a,N(t))\phi _1(a)\hbox {d}a\). We also define a nonlinear operator \(F: \mathbf {X}_+\rightarrow \mathbf {X}\) by
Based on Assumption (\(\text{ H }_1\)), it is not difficult to prove that the operator F is Lipschitz continuous and there exists a positive constant \(r>0\) such that
where I denotes the identity operator. The proof of this result can be referred to Inaba (2006).
Set \(u(t)=(P(t,\cdot ),Q(t,\cdot ))^T\). Then system (2.1)–(2.3) can be formulated as a nonlinear Cauchy problem on the Banach space \(\mathbf {X}\):
where \(u_0(a)=(P_0(a),Q_0(a))^\mathrm{T}\). We can see that operator A generates a \(C_0\)-semigroup \(\{\hbox {e}^{tA}\}_{t\geqslant 0}\) and there exist numbers \(M\geqslant 1\) and \(\alpha >0\) such that
Let \(r>0\) be a constant such that (A.3) holds. Using this r and according to Busenberg et al. (1991), abstract Cauchy problem (A.4) can be rewritten as
Investigating problem (A.6), we obtain the mild solution by the solution of the integral equation
Let \(\{S(t)\}_{t\geqslant 0}\) be the semiflow defined by the solutions of the above variation of constants formula. Then, \(S(t)u_0\) can be given as the limit of the iterative sequence \(\{u^n\}_{n\geqslant 0}\) such that
Notice that \(u^{n+1}\) is a linear convex combination of \(\hbox {e}^{tA}u_0\in \mathbf {X}_+\) and \(\hbox {e}^{(t-s)A}(I+rF)u^n\in \mathbf {X}_+\). Then, based on the positivity of \(\hbox {e}^{tA}\) and \(I+rF\), we conclude that \(u^{n+1}\in \mathbf {X}_+\) if \(u^n\in \mathbf {X}_+\) by applying (A.3). It follows from the Lipschitz continuity of F that \(\{u^n\}\) converges to the mild solution \(S(t)u_0\in \mathbf {X}_+\) uniformly. Applying (A.5), we have the estimate
where \(K:=||I+rF||\). From the Gronwall inequality, we can estimate that:
Because the norm of the local solution grows at most exponentially as time evolves, it can be extended to a global one. Hence, the solution \(S(t)u_0, t > 0,\) is global.
Finally, we say that Cauchy problem (A.4) has a unique mild solution \(S(t)u_0\in \mathbf {X}_+\) for each \(u_0\in \mathbf {X}_+\), and \(\mathbf {X}_+\) is positively invariant with respect to the semiflow \(\{S(t)\}_{t\geqslant 0}\).
Rights and permissions
About this article
Cite this article
Liu, Z., Chen, J., Pang, J. et al. Modeling and Analysis of a Nonlinear Age-Structured Model for Tumor Cell Populations with Quiescence. J Nonlinear Sci 28, 1763–1791 (2018). https://doi.org/10.1007/s00332-018-9463-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00332-018-9463-0