Abstract
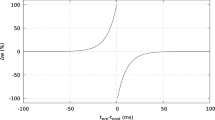
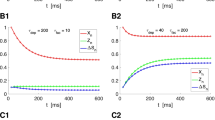
Spike-timing dependent plasticity (STDP) is a type of synaptic modification found relatively recently, but the underlying biophysical mechanisms are still unclear. Several models of STDP have been proposed, and differ by their implementation, and in particular how synaptic weights saturate to their minimal and maximal values. We analyze here kinetic models of transmitter-receptor interaction and derive a series of STDP models. In general, such kinetic models predict progressive saturation of the weights. Various forms can be obtained depending on the hypotheses made in the kinetic model, and these include a simple linear dependence on the value of the weight (“soft bounds”), mixed soft and abrupt saturation (“hard bound”), or more complex forms. We analyze in more detail simple soft-bound models of Hebbian and anti-Hebbian STDPs, in which nonlinear spike interactions (triplets) are taken into account. We show that Hebbian STDPs can be used to selectively potentiate synapses that are correlated in time, while anti-Hebbian STDPs depress correlated synapses, despite the presence of nonlinear spike interactions. This correlation detection enables neurons to develop a selectivity to correlated inputs. We also examine different versions of kinetics-based STDP models and compare their sensitivity to correlations. We conclude that kinetic models generally predict soft-bound dynamics, and that such models seem ideal for detecting correlations among large numbers of inputs.
Similar content being viewed by others
References
Abarbanel HD, Gibb L, Huerta R, Rabinovich MI (2003) Biophysical model of synaptic plasticity dynamics. Biol Cybern 89:214–226
Abeles M (1991) Corticonics: neural circuits of the cerebral cortex. Cambridge University Press, Cambridge
Anwyl R (2006) Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 78:17–37
Badoual M, Zou Q, Davison AP, Rudolph M, Bal T, Frégnac Y, Destexhe A (2006) Biophysical and phenomenological models of multiple spike interactions in spike-timing dependent plasticity. Int J Neural Syst 16:79–97
Bell CC, Han VZ, Sugawara Y, Grant K (1997) Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387:278–281
Bi GQ, Poo MM (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength and postsynaptic cell type. J Neurosci 18:10464–10472
Burkitt AN, Meffin H, Grayden DB (2004) Spike-timing-dependent plasticity: the relationship to rate-based learning for models with weight dynamics determined by a stable fixed point. Neural Comput 16:885–940
Castellani GC, Quinlan EM, Cooper LN, Shouval HZ (2001) A biophysical model of bidirectional synaptic plasticity: dependence on ampa and nmda receptors. Proc Nat Acad Sci USA 98:12772–12777
de Charms RC, Merzenich MM (1996) Primary cortical representation of sounds by the coordination of action-potential timing. Nature 381:610–613
Dan Y, Poo MM (2006) Spike timing-dependent plasticity: from synapse to perception. Physiol Rev 86:1033–1048
Debanne D, Gahwiler BH, Thompson SM (1994) Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc Natl Acad Sci USA 91:1148–1152
Destexhe A, Paré D (1999) Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81:1531–1547
Destexhe A, Mainen ZF, Sejnowski TJ (1994) An efficient method for computing synaptic conductances based on a kinetic model of receptor binding. Neural Comput 6:10–14
Destexhe A, Mainen ZF, Sejnowski TJ (1998) Kinetic models of synaptic transmission. In: Methods in neuronal modeling, chapter 1, MIT Press, Cambridge, Massachusetts, pp 1–26
Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ (2001) Fluctuating synaptic conductances recreate in-vivo-like activity in neocortical neurons. Neurosci 107:13–24
Destexhe A, Rudolph M, Paré D (2003) The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4:739–751
Duguid I, Sjostrom PJ (2006) Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol 16:312–322
Froemke RC, Dan Y (2002) Spike timing-dependent synaptic modification induced by natural spike trains. Nature 416:433–438
Gerstner W, Kistler W (2002) Spiking neuron models. Cambridge University Press, Cambridge
Gerstner W, Kempter R, van Hemmen JL, Wagner H (1996) A neuronal learning rule for sub-millisecond temporal coding. Nature 383:76–81
Gütig R, Aharonov R, Rotter S, Sompolinsky H (2003) Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J Neurosci 23:3697–3714
Hebb DO (1949) The organization of behavior. John Wesley & Sons, New York
Hines ML, Carnevale NT (1997) The neuron simulation environment. Neural Comput 9:1179–1209
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117:500–544
Izhikevich EM, Desai NS (2003) Relating STDP to BCM. Neural Comput 15:1511–1523
Karbowski J, Ermentrout GB (2002) Synchrony arising from a balanced synaptic plasticity in a network of heterogeneous neural oscillators. Phys Rev E 65:031902
Karmarkar UR, Buonomano DV (2002) A model of spike-timing dependent plasticity: one or two coincidence detectors? J Neurophysiol 88:507–513
Kistler WM, van Hemmen JL (2000) Modeling synaptic plasticity in conjuction with the timing of pre- and postsynaptic action potentials. Neural Comput 12:385–405
Levy WB, Steward O (1983) Temporal contiguity requirements for long-term associative potentiation/depression in hippocampus. Neurosci 8:791–797
Magee JC, Johnston DA (1997) A synaptically controlled, associative signal for hebbian plasticity in hippocampal neurons. Science 275:209–213
Markram H, Lübke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275:213–215
Montgomery JM, Pavlidis P, Madison DV (2001) Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron 29:691–701
Morrison A, Aertsen A and Diesmann M (2007) Spike-timing dependent plasticity in balanced random networks. Neural Comput (in press)
Nicoll RA (2003) Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B 358: 721–726
Paré D, Shink E, Gaudreau H, Destexhe A, Lang E (1998) Impact of spontaneous synaptic activity on the resting properties of cat neocortical neurons in vivo. J Neurophysiol 79:1450–1460
Pfister JP, Gerstner W (2006) Triplets of spikes in a model of spike timing-dependent plasticity. J Neurosci 26:9673–9682
Riehle A, Grun S, Diesmann M, Aertsen A (1997) Spike synchronization and rate modulation differentially involved in motor cortical function. Science 278:1950–1953
Roche KW, Tingley WG, Huganir RL (1994) Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol 4:383–388
van Rossum MC, Bi GQ, Turrigiano GG (2000) Stable Hebbian learning from spike timing-dependent plasticity. J Neurosci 20:2211–2221
Rubin J, Lee DD, Sompolinsky H (2001) Equilibrium properties of temporally asymmetric Hebbian plasticity. Phys Rev Lett 86:364–367
Rubin JE, Gerkin RC, Bi G-Q, Chow CC (2005) Calcium time course as a signal for spike-timing-dependent plasticity. J Neurophysiol 93:2600–2613
Rudolph M, Destexhe A (2001) Do neocortical pyramidal neurons display stochastic resonance? J Comput Neurosci 11:19–42
Senn W, Markram H, Tsodyks M (2001) An algorithm for modifying neurotransmitter release probability based on pre- and postsynaptic spike timing. Neural Comput 13:35–67
Shouval HZ, Kalantzis G (2005) Stochastic properties of synaptic transmission affect the shape of spike time-dependent plasticity curves. J Neurophysiol 93:1069–1073
Shouval HZ, Bear MF, Cooper LN (2002) A unified model of nmda receptor-dependent bidirectional synaptic plasticity. Proc Nat Acad Sci USA 99:10831–10836
Sjöström J, Turrigiano GG, Nelson SB (2001) Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32:1149–1164
Soderling TR, Derkach VA (2000) Postsynaptic protein phosphorylation and LTP. Trends Neurosci 23:75–80
Song S, Abbott LF (2001) Cortical development and remapping through spike timing-dependent plasticity. Neuron 32:339–350
Song S, Miller KD, Abbott LF (2000) Competitive Hebbian learning through spike-timing dependent synaptic plasticity. Nat Neurosci 3:919–926
Steriade M, Timofeev I, Grenier F (2001) Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85:1969–1985
Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H, Aertsen A (1995) Dynamics of neuronal interactions in monkey cortex in relation to behavioural events. Nature 373:515–518
Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L (2005) Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol 32:237–249
Zohary E, Shadlen MN, Newsome WT (1994) Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370:140–143
Zou Q, Rudolph M, Roy N, Sanchez-Vives M, Contreras D, Destexhe A. Reconstructing synaptic background activity from conductance measurements in vivo. Neurocomputing 65:673–678
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, Q., Destexhe, A. Kinetic models of spike-timing dependent plasticity and their functional consequences in detecting correlations. Biol Cybern 97, 81–97 (2007). https://doi.org/10.1007/s00422-007-0155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-007-0155-3