Abstract
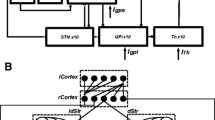
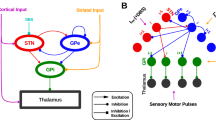
Recordings from the basal ganglia’s subthalamic nucleus are acquired via microelectrodes immediately prior to the application of Deep Brain Stimulation (DBS) treatment for Parkinson’s Disease (PD) to assist in the selection of the final point for the implantation of the DBS electrode. The acquired recordings reveal a persistent characteristic beta band peak in the power spectral density function of the Local Field Potential (LFP) signals. This peak is considered to lie at the core of the causality–effect relationships of the parkinsonian pathophysiology. Based on LFPs acquired from human subjects during DBS for PD, we constructed a computational model of the basal ganglia on the population level that generates LFPs to identify the critical pathophysiological alterations that lead to the expression of the beta band peak. To this end, we used experimental data reporting that the strengths of the synaptic connections are modified under dopamine depletion. The hypothesis that the altered dopaminergic modulation may affect both the amplitude and the time course of the postsynaptic potentials is validated by the model. The results suggest a pivotal role of both of these parameters to the pathophysiology of PD.
Similar content being viewed by others
References
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375
Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK (2004) Dopamine modulates release from corticostriatal terminals. J Neurosci 24: 9541–9552
Barchas JD, Akil H, Elliott GR, Holman RB, Watson SJ (1978) Behavioral neurochemistry: neuroregulators and behavioral states. Science 200: 964–973
Baufreton J, Zhu ZT, Garret M, Bioulac B, Johnson SW, Taupignon AI (2005) Dopamine receptors set the pattern of activity generated in subthalamic neurons. FASEB J 19: 1771–1777
Bauswein E, Fromm C, Preuss A (1989) Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res 493: 198–203
Beckstead RM, Wooten GF, Trugman JM (1988) Distribution of D1 and D2 dopamine receptors in the basal ganglia of the cat determined by quantitative autoradiography. J Comp Neurol 268: 131–145
Bedard C, Kroger H, Destexhe A (2006) Model of low-pass filtering of local field potentials in brain tissue. Phys Rev 73: 051911
Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J (1991) Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: 403–406
Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid A-L (2002) Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord 17: S145–S149
Bergman H, Deuschl G (2002) Pathophysiology of Parkinson’s disease: from clinical neurology to basic neuroscience and back. Mov Disord 17: S28–S40
Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ (2002) Move to the rhythm: oscillations in the subthalamic nucleus–external globus pallidus network. Trends Neurosci 25: 525–531
Bolam JP, Hanley JJ, Booth PAC, Bevan MD (2000) Synaptic organization of the basal ganglia. J Anat 196: 527–542
Boraud T, Brown P, Goldberg JA, Graybiel AM, Magill PJ (2005) Oscillations in the basal ganglia: the good, the bad, and the unexpected. Basal Ganglia VIII: 3–24
Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18: 357–363
Brown P, Williams D (2005) Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 116: 2510–2519
Brown P, Kupsch A, Magill PJ, Sharott A, Harnack D, Meissner W (2002) Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol 177: 581–585
Bullock TH (1997) Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc Natl Acad Sci USA 94: 1–6
Calabresi P, Centonze D, Bernardi G (2000) Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci 23: 57–63
Carpenter MB, Carleton SC, Keller JT, Conte P (1981) Connections of the subthalamic nucleus in the monkey. Brain Res 224: 1–29
Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS (2008) Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci 27: 671–682
Charara A, Sidibe M, Smith Y (2003) Basal ganglia circuitry and synaptic connectivity. In: Tarsy D, Vitek JL, Lozano AM (eds) Contemporary clinical neurology: surgical treatment of Parkinson’s disease and other movement disorders. Humana Press, pp 19–39
Chen CC, Pogosyan A, Zrinzo LU, Tisch S, Limousin P, Ashkan K, Yousry T, Hariz MI, Brown P (2006) Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’s disease surgery. Exp Neurol 198: 214–221
Choe Y, Miikkulainen R (2003) The role of postsynaptic potential decay rate in neural synchrony. Neurocomputing 52–54: 707–712
Cooper AJ, Stanford IM (2000) Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro. J Physiol 527: 291–304
Cooper AJ, Stanford IM (2001) Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABAA IPSCs in vitro. Neuropharmacology 41: 62–71
Cosandier-Rimele D, Badier JM, Chauvel P, Wendling F (2007) A physiologically plausible spatio-temporal model for EEG signals recorded with intracerebral electrodes in human partial epilepsy. IEEE Trans Biomed Eng 54: 380–388
Czubayko U, Plenz D (2002) Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci 99: 15764–15769
Dostrovsky J, Bergman H (2004) Oscillatory activity in the basal ganglia—relationship to normal physiology and pathophysiology. Brain 127: 721–722
Elson RC, Selverston AI, Abarbanel HDI, Rabinovich MI (2002) Inhibitory synchronization of bursting in biological neurons: dependence on synaptic time constant. J Neurophysiol 88: 1166–1176
Falls WM, Park MR, Kitai ST (1983) An intracellular HRP study of the rat globus pallidus. II. Fine structural characteristics and synaptic connections of medially located large GP neurons. J Comp Neurol 221: 229–245
Floran B, Floran L, Erlij D, Aceves J (2004) Dopamine D4 receptors inhibit depolarization-induced [3H]GABA release in the rat subthalamic nucleus. Eur J Pharmacol 498: 97–102
Freeman WJ (1978) Models of the dynamics of neural populations. Electroencephalogr Clin Neurophysiol 34: 9–18
Freeman WJ (2000) Characteristics of the synchronization of brain activity imposed by finite conduction velocities of axons. Int J Bifurc Chaos 10: 2307–2322
Gerstner W, Kistler W (2002) Spiking neuron models: an introduction. Cambridge University Press, New York
Gillies A, Willshaw D (2004) Models of the subthalamic nucleus. The importance of intranuclear connectivity. Med Eng Phys 26: 723–732
Gopalsamy K, Leung I (1996) Delay induced periodicity in a neural netlet of excitation and inhibition. Physica D 89: 395–426
Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P (1997) Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci 17: 204–215
Gurney K, Prescott TJ, Redgrave P (2001) A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern 84: 401–410
Haber SN (2008) Functional anatomy and physiology of the basal ganglia: non-motor functions. In: Tarsy D, Vitek JL, Starr PA, Okun MS (eds) Deep brain stimulation in neurological and psychiatric disorders. Humana Press, pp 33–62
Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM (2004) The subthalamic nucleus in the context of movement disorders. Brain 127: 4–20
Hammond C, Bergman H, Brown P (2007) Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30: 357–364
Humphries MD, Stewart RD, Gurney KN (2006) A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci 26: 12921–12942
Jansen BH, Rit VG (1995) Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol Cybern 73: 357–366
Jansen BH, Zouridakis G, Brandt ME (1993) A neurophysiologically-based mathematical model of flash visual evoked potentials. Biol Cybern 68: 275–283
Johnson PI, Napier TC (1997) GABA-and glutamate-evoked responses in the rat ventral pallidum are modulated by dopamine. Eur J Neurosci 9: 1397–1406
Johnston D, Wu SM (1995) Foundations of cellular neurophysiology. MIT Press, Cambridge
Kincaid AE, Zheng T, Wilson CJ (1998) Connectivity and convergence of single corticostriatal axons. J Neurosci 18: 4722–4731
Kita H, Kitai ST (1991) Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res 564: 296–305
Kita H, Kita T, Kitai ST (1985) Active membrane properties of rat neostriatal neurons in an in vitro slice preparation. Exp Brain Res 60: 54–62
Kreiss DS, Mastropietro CW, Rawji SS, Walters JR (1997) The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson’s disease. J Neurosci 17: 6807–6819
Kuhn AA, Kupsch A, Schneider GH, Brown P (2006) Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 23: 1956–1960
Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI et al (2008) High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 28: 6165–6173
Lange KW, Kornhuber J, Riederer P (1997) Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev 21: 393–400
Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL (1995) Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345: 91–95
Liu X (2003) What can be learned from recording local field potentials from the brain via implanted electrodes used to treat patients with movement disorders. Curr Med Lit 19: 1–6
Logothetis NK, Kayser C, Oeltermann A (2007) In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron 55: 809–823
Lopes da Silva FH, Hoeks A, Smits H, Zetterberg LH (1974) Model of brain rhythmic activity. Biol Cybern 15: 27–37
Lopes da Silva FH, van Rotterdam A, Barts P, van Heusden E, Burr W (1976) Models of neuronal populations: the basic mechanisms of rhythmicity. Prog Brain Res 45: 281–308
Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ (2008) Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci 28: 4795
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189–225
Nakanishi H, Kita H, Kitai ST (1987) Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res 437: 35–44
Nakanishi H, Kita H, Kitai ST (1990) Intracellular study of rat entopeduncular nucleus neurons in an in vitro slice preparation: electrical membrane properties. Brain Res 527: 81–88
Nambu A (2005) A new approach to understand the pathophysiology of Parkinson’s disease. J Neurol 252(Suppl 4): IV/1–IV/4
Nambu A, Llinas R (1994) Electrophysiology of globus pallidus neurons in vitro. J Neurophysiol 72: 1127–1139
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43: 111–117
Nunez PL, Srinivasan R (2006) Electric fields of the brain: the neurophysics of EEG. Oxford University Press, Oxford
Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC (2000) Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience 97: 79–88
Parent A (1986) Comparative neurobiology of the basal ganglia. Wiley, New York
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The corticobasal gangliathalamocortical loop. Brain Res Rev 20: 91127
Penney JB, Young AB (1983) Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci 6: 73–94
Pollack AE (2001) Anatomy, physiology, and pharmacology of the basal ganglia. Neurol Clin 19: 523–534
Priori A, Egidi M, Pesenti A, Rohr M, Rampini P, Locatelli M, Tamma P, Caputo E, Chiesa V, Barbieri S (2003) Do intraoperative microrecordings improve subthalamic nucleus targeting in stereotactic neurosurgery for Parkinson’s disease? J Neurosurg Sci 47: 56–60
Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM (2004) Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol 189: 369–379
Ravenscroft P, Brotchie J (2000) NMDA receptors in the basal ganglia. J Anat 196: 577–585
Rinzel J, Terman D, Wang XJ, Ermentrout B (1998) Propagating activity patterns in large-scale inhibitory neuronal networks. Science 279: 1351–1355
Sato F, Parent M, Levesque M, Parent A (2000) Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol 424: 142–152
Seamans J, Durstewitz D (2008) Dopamine modulation. Scholarpedia 3: 2711
Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P (2005) Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci 21: 1413–1422
Shen KZ, Johnson SW (2000) Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol 525: 331–341
Shen KZ, Johnson SW (2006) Subthalamic stimulation evokes complex EPSCs in the rat substantia nigra pars reticulata in vitro. J Physiol 573: 697–709
Shen KZ, Zhu ZT, Munhall A, Johnson SW (2003) Dopamine receptor supersensitivity in rat subthalamus after 6-hydroxydopamine lesions. Eur J Neurosci 18: 2967–2974
Shink E, Smith Y (1995) Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA-and glutamate-containing terminals in the squirrel monkey. J Comp Neurol 358: 119–141
Smith Y, Kieval JZ (2000) Anatomy of the dopamine system in the basal ganglia. Trends Neurosci 23: 28–33
Smith Y, Wichmann T (2008) Functional anatomy and physiology of the basal ganglia: motor functions. In: Tarsy D, Vitek JL, Starr PA, Okun MS (eds) Deep brain stimulation in neurological and psychiatric disorders. Humana Press, pp 1–32
Smith Y, Bevan MD, Shink E, Bolam JP (1998) Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86: 353–387
Terman D, Rubin JE, Yew AC, Wilson CJ (2002) Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci 22: 2963–2976
Trottenberg T, Kupsch A, Schneider GH, Brown P, Kuhn AA (2007) Frequency-dependent distribution of local field potential activity within the subthalamic nucleus in Parkinson’s disease. Exp Neurol 205: 287–291
Utter AA, Basso MA (2008) The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev 32: 333–342
Van Albada SJ, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. I Firing rates in healthy and parkinsonian states. J Theor Biol 257: 642–663
Van Rotterdam A, Lopes da Silva FH, Van den Ende J, Viergever MA, Hermans AJ (1982) A model of the spatial-temporal characteristics of the alpha rhythm. Bull Math Biol 44: 283–305
Vibert JF, Parkdaman K, Azmy N (1994) Interneural delay modification synchronizes biologically plausible neural networks. Neural Netw 7: 589–607
Vibert JF, Alvarez F, Pham J (1998) Effects of transmission delays and noise in recurrent excitatory neural networks. Biosystems 48: 255–262
Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA (2006) Phase relationship support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience 144: 762–776
Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO (2006) Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol 96: 3248–3256
Wendling F, Bellanger JJ, Bartolomei F, Chauvel P (2000) Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biol Cybern 83: 367–378
Wendling F, Bartolomei F, Bellanger JJ, Chauvel P (2002) Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur J Neurosci 15: 1499–1508
West AR, Grace AA (2002) Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22: 294
Wichmann T, DeLong MR (2006) Basal ganglia discharge abnormalities in Parkinson’s disease. J Neural Transm [Suppl] 70: 21–25
Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM (2006) Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson’s disease. Exp Neurol 197: 244–251
Yasumoto S, Tanaka E, Hattori G, Maeda H, Higashi H (2002) Direct and indirect actions of dopamine on the membrane potential in medium spiny neurons of the mouse neostriatum. J Neurophysiol 87: 1234–1243
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsirogiannis, G.L., Tagaris, G.A., Sakas, D. et al. A population level computational model of the basal ganglia that generates parkinsonian local field potential activity. Biol Cybern 102, 155–176 (2010). https://doi.org/10.1007/s00422-009-0360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-009-0360-3